Urinary excretion of β2-microglobulin as a prognostic marker in immunoglobulin A nephropathy
Article information
Abstract
Background/Aims
β2-microglobulin (β2-MG) is freely filtered at the glomerulus and subsequently reabsorbed and catabolized by proximal renal tubular cells. Urinary β2-MG is an early and sensitive biomarker of acute kidney injury; however, its utility as a biomarker of immunoglobulin A nephropathy (IgAN) is unclear.
Methods
We included urinary β2-MG levels in the routine laboratory examination of all inpatients with biopsy-proven IgAN at our hospital from 2006 to 2010. We retrospectively analyzed the correlation between β2-MG levels and clinical parameters as a prognostic biomarker of IgAN.
Results
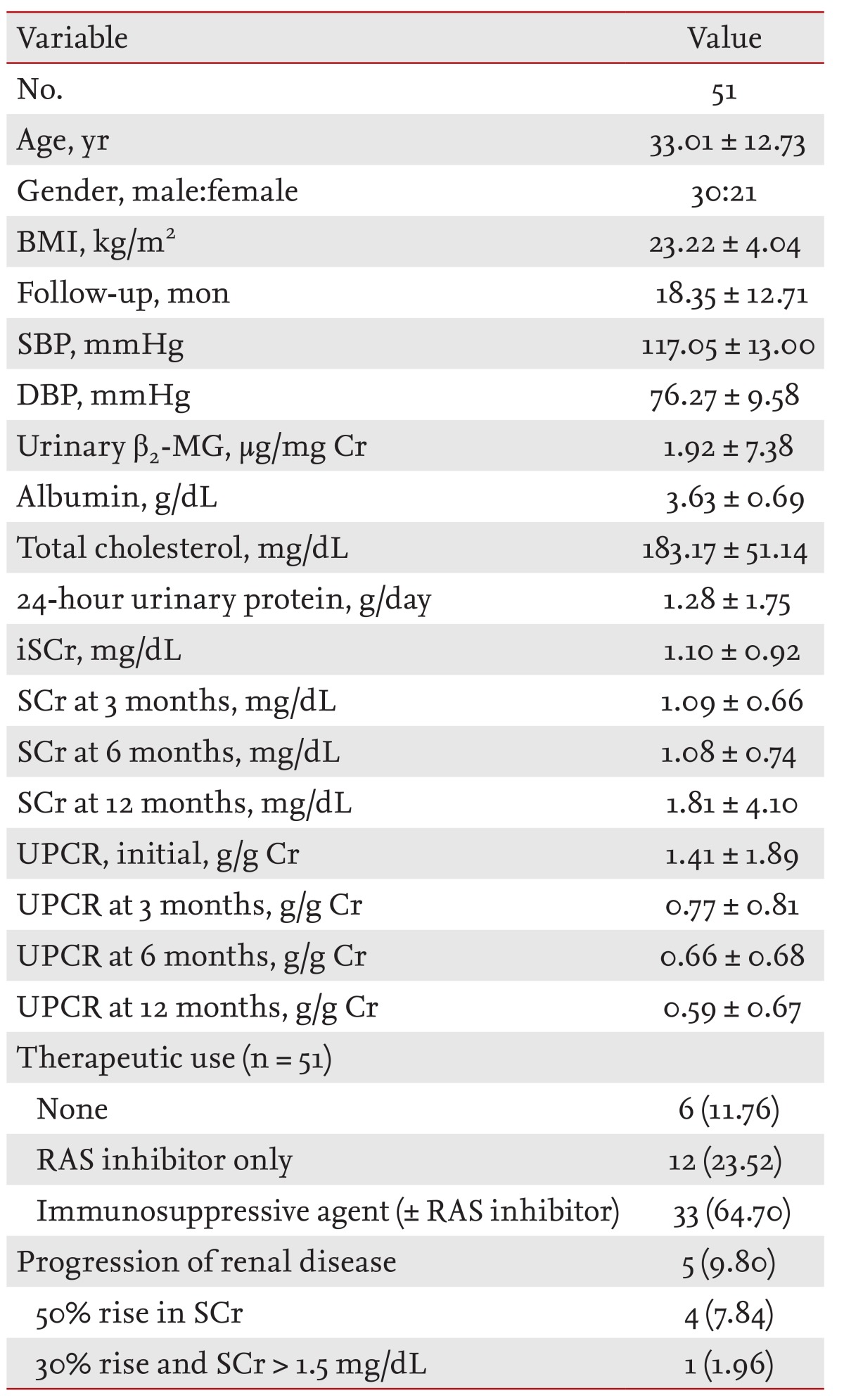
A total of 51 patients (30 males, 21 females; mean age, 33.01 ± 12.73 years) with IgAN were included in this study. Initial demographic, clinical, and laboratory data for all patients are listed. The mean initial estimated glomerular filtration rate and 24-hour urine protein levels were 94.69 ± 34.78 mL/min/1.73 m2 and 1.28 ± 1.75 g/day, respectively. The mean level of urinary β2-MG was 1.92 ± 7.38 µg/mg creatinine. There was a significant correlation between initial serum creatinine (iSCr), urine protein creatinine ratio (UPCR), and the level of β2-MG (r = 0.744, r = 0.667, p < 0.01). There was also a significant correlation between renal function tests and the level of urinary β2-MG (p < 0.01). Cox regression analysis showed that albumin, β2-MG, iSCr, and UPCR were significant predictors of disease progression in IgAN.
Conclusions
Urinary β2-MG levels showed a significant correlation with renal function and proteinuria in IgAN. Thus, we propose that urinary β2-MG may be an additional prognostic factor in patients with IgAN.
INTRODUCTION
Immunoglobulin A nephropathy (IgAN) is the most common primary glomerulonephritis both in Korea and worldwide [1]. The clinical spectrum covers a wide range of features from minor urinary abnormalities (asymptomatic hematuria and mild proteinuria with normal renal function) to acute and chronic renal insufficiency. Approximately 15% to 40% of patients eventually progress to end-stage renal disease (ESRD) despite an initially benign prognosis [2]. Accurate prediction of prognosis at the time of initial diagnosis is necessary to optimize treatment.
Several variables have been identified as predictors of prognosis, including elevated serum creatinine (SCr) concentrations, severe proteinuria, arterial hypertension, and histological findings such as tubular damage [3,4].
A number of reports have emphasized the prognostic value of glomerular changes with focal segmental glomerulosclerosis, crescents (Cs) or diffuse mesangial cell proliferation, and tubulointerstitial changes in IgAN [5]. In addition, tubular injury grading predicted renal outcomes better than other histological parameters [6].
Urinary β2-microglobulin (β2-MG) is an early and sensitive biomarker of acute kidney injury. β2-MG is freely filtered at the glomerulus and subsequently reabsorbed and catabolized by proximal renal tubular cells and is not normally detected in urine [7]. Urinary β2-MG is a marker of tubulointerstitial injury and predicts the risk of developing ESRD in idiopathic membranous nephropathy [8]. However, whether β2-MG is a useful prognostic biomarker of IgAN is unclear.
We investigated associations between urinary β2-MG levels and clinical parameters as well as histological changes in IgAN. We determined whether the excretion of β2-MG provides additional prognostic information in patients with IgAN.
METHODS
Study selection
Fifty-six patients with biopsy-proven IgAN were enrolled in this study. To ensure an adequate follow-up period, patients followed up less than 6 months were excluded from the analysis. Thus, a total of 51 patients (30 males, 21 females; mean age, 33.01 ± 12.73 years) were included in this study. We measured urinary β2-MG levels of 51 patients before treatment at the Kyung Hee University Medical Center from 2006 to 2010. After baseline measurements, patients were transferred to our medical center for treatment. The median follow-up period was 18.35 ± 12.71 months.
Baseline measurement
Gender, age, body weight and height, systolic blood pressure (SBP), diastolic blood pressure (DBP), SCr, urine protein creatinine ratio (UPCR), albumin, and total cholesterol were recorded at the time of the prerenal biopsy. A 24-hour urine sample was obtained for measurement of creatinine (Cr) and total protein. The therapeutic use of angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB), prednisolone, and prednisolone (± other immunosuppressive agent) combined with ACEi/ARB were recorded. After baseline measurements, we collected data on SCr, urine protein and Cr at 3, 6, and 12 months. Then, we analyzed the clinical correlations between urinary levels of β2-MG, other clinical parameters, and histological findings in IgAN.
Calculations and definitions
Body mass index (BMI) was calculated from baseline body weight and height measurements. Estimated glomerular filtration rate (eGFR) was calculated using the modification of diet in the renal disease study equation. First morning urine samples were collected on the day of biopsy and stored at -70℃. Proteinuria was expressed as urinary protein/Cr ratio (g/g Cr, UPCR).
We defined immunosuppressive treatment (± renin-angiotensin system [RAS]) as prednisolone or other immunosuppressive agent (cyclophosphamide, cyclosporine, azathioprine) ± ACEi/ARB.
Pathologic staging included H.S. Lee's grading, tubulointerstitial inflammation (TII), and tubulointerstitial fibrosis (TIF) grading. H.S. Lee's grading system for IgAN was defined as follows: grade I, normal or focal mesangial cell proliferation; grade II, diffuse mesangial cell proliferation or < 25% of glomeruli with Cs, segmental sclerosis (SS), and global sclerosis (GS); grade III, 25% to 49% of glomeruli with Cs/SS/GS; grade IV, 50% to 75% of glomeruli with Cs/SS/GS; and grade V, > 75% of glomeruli with Cs/SS/GS. TII and TIF grading systems for IgAN were defined as follows: grade 0, absent; grade 1, focal segmental; grade 2, mild diffuse; grade 3, moderate diffuse; and grade 4, marked diffuse.
We defined renal progression as: 1) a rise in SCr > 50% above baseline measurements and 2) a rise in SCr > 30% and an absolute level > 1.5 mg/dL.
Statistical analysis
Bivariate correlation and linear regression analyses were used to compare categorical and continuous variables between β2-MG and clinical parameters. Independent variables were tested in linear univariate regression analyses. These analyses included age, BMI, SBP/DBP, albumin, initial UPCR (iUPCR), SCr and H.S. Lee, TIF, and TII grading. Independent variables with a p < 0.1 in univariate linear regression analyses were retested in the multivariate regression model. We also used Cox regression analysis for analyzing clinical variables with disease progression in IgAN. Possible collinearity for univariate significant predictors was evaluated. A backward stepwise selection algorithm with criteria for exclusion and likelihood ratio test with a p value greater than 0.10 and smaller than 0.05 for inclusion were used.
RESULTS
Baseline clinical and laboratory findings in IgAN patients
A total of 51 patients (30 males, 21 females; mean age, 33.01 ± 12.73 years) with IgAN were included in this study. Initial demographic, clinical, and laboratory data of the 51 patients are listed in Table 1. Median duration of follow-up was 18.35 ± 12.71 months. In a minority of patients (13.7%), proteinuria of > 2.0 g/day with an eGFR < 60 mL/min/1.73 m2 was observed. The mean initial serum creatinine (iSCr) was 1.10 ± 0.92 mg/dL. The mean level of iUPCR and urinary β2-MG was 1.41 ± 1.89 g/g Cr and 1.92 ± 7.38 µg/mg Cr, respectively.
During follow-up, patients were treated with immunosuppressive agents with and without RAS inhibitor (n = 33, 64.70%). The distribution of immunosuppressive agents was prednisolone ± ACEi/ARB (33.3%), prednisolone + other immunosuppressive agent ± ACEi/ARB (17.6%), and prednisolone alone (13.7%).
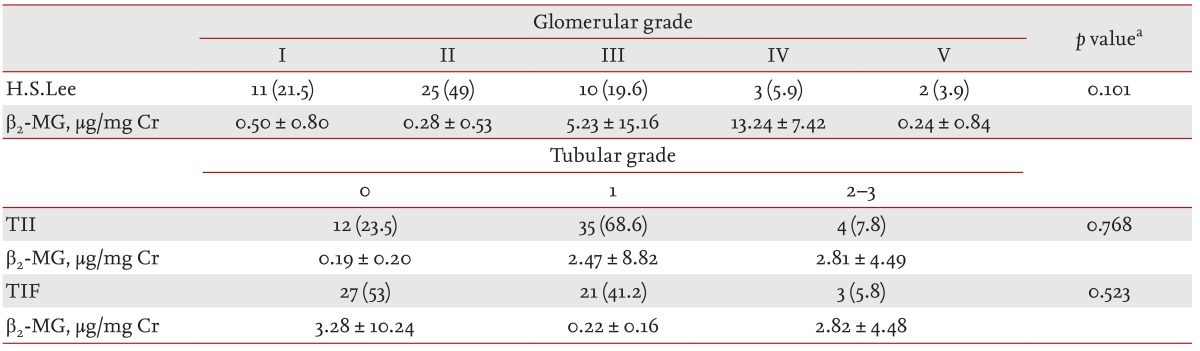
Table 2 shows the β2-MG values for each pathological grade of IgAN. No statistical difference with respect to β2-MG was observed between different grades. The distribution in glomerular grades of the 51 patients using the H.S. Lee grading was as follows: grade I, 11 patients (21.5%); grade II, 25 patients (49%); grade III, 10 patients (19.6%); grade IV, three patients (5.9%); grade V, two patients (3.9%). In addition, the tubular grade results when using the TII and TIF grading systems are shown in Table 2.
Association of urinary β2-MG with clinical parameters
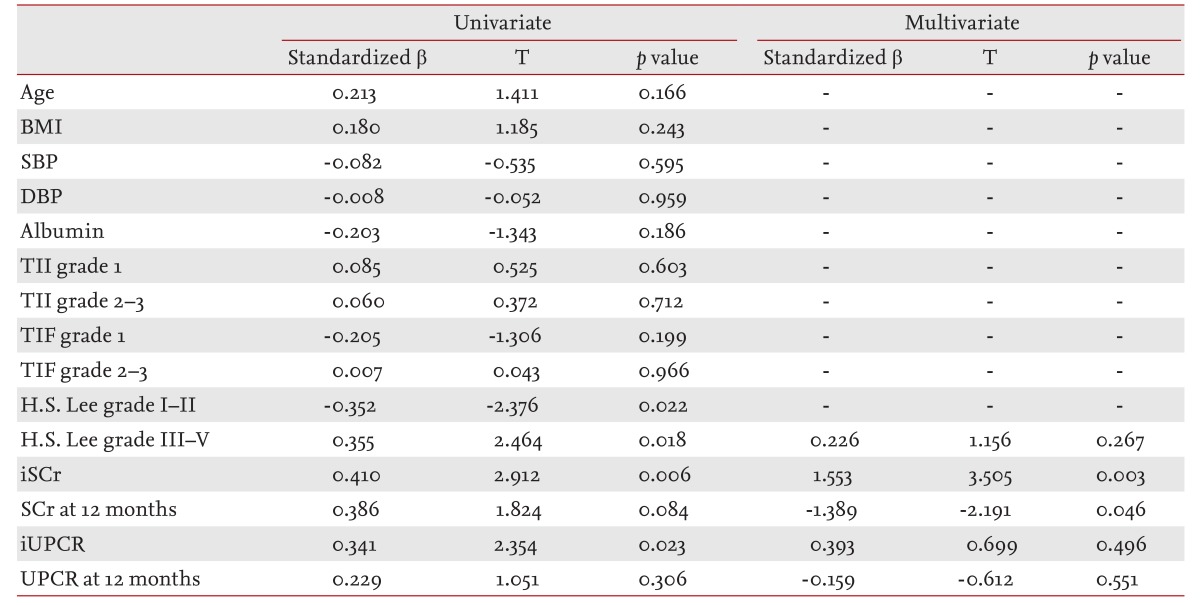
In univariate linear regression analysis, there was a significant correlation between iSCr (β = 0.410, p = 0.006), iUPCR (β = 0.341, p = 0.023) and the level of urinary β2-MG. Multivariate linear regression analysis showed iSCr (β = 1.553, p = 0.003) and SCr12 m (β = -1.389, p = 0.046) were independently associated with β2-MG (Table 3). Fig. 1 is a scatter plot representing the association with β2-MG, iSCr, and iUPCR. Both iSCr and iUPCR correlated significantly with β2-MG (r = 0.744, p < 0.01 and r = 0.667, p < 0.01, respectively). Fig. 2 shows the comparison of urine β2-MG based on the iSCr and iUPCR. When patients with IgAN were categorized according to SCr (1.2 mg/dL) and UPCR (1.0 g/g Cr), patients with > 1.2 mg/dL of SCr and > 1.0 g/g Cr of UPCR had higher urine β2-MG levels (p < 0.01) (Fig. 2).
Univariate and multivariate linear regression analyses of associations of clinical variables with urinary β2-microglobulin in immunoglobulin A nephropathy

Correlations between urinary β2-microglobulin (β2-MG). (A) Initial serum creatinine (iSCr), and (B) initial urine protein creatinine ratio (iUPCR) in patients with immunoglobulin A nephropathy (p < 0.01).

Urinary β2-microglobulin (β2-MG) levels in immunoglobulin A nephropathy according to initial serum creatinine (iSCr), initial urine protein creatinine ratio (iUPCR). aStatistical significance p < 0.01.
Although β2-MG is a tubular injury marker, there was a positive correlation between β2-MG and H.S. Lee grade in univariate linear analysis. Conversely, we found no association between β2-MG and tubular TII and TIF injury grades (Table 3).
Urinary β2-MG as a predictor of renal outcomes in patients with IgAN
Of the 51 patients, five had disease progression. In four patients, SCr concentration increased by > 50% and in one patient SCr concentration increased > 30% with an absolute level > 1.5 mg/dL (Table 1).
β2-MG, albumin, iSCR, and UPCR were significant predictors of progression in univariate Cox regression analysis (Table 4).
DISCUSSION
Recent advancements in molecular analysis have resulted in the identification of a wide range of potential serum and urine biomarkers for assessing renal function and injury as well as predicting the development of kidney disease.
Several studies have shown that urinary β2-MG is a marker of tubulointerstitial injury, predicts the risk of ESRD in idiopathic membranous nephropathy [8] and was identified as a potential biomarker of acute renal allograft rejection [9].
In idiopathic membranous nephropathy, histological markers appeared to be of limited value, whereas the severity of proteinuria and increased SCr concentration are the strongest predictors of outcome. Additionally, urinary excretion of α2-MG, β2-MG, and IgG are prognostic indicators of idiopathic membranous nephropathy [8]; however, their utility as prognostic indicators of IgAN is unclear.
Our data indicate that urinary excretion of β2-MG is significantly correlated with iSCr and UPCR (Fig. 1). Urinary β2-MG excretion significantly increased with iSCr measurements ≥ 1.2 mg/dL or with an iUPCR ≥ 1.0 g/g Cr (p < 0.01) (Fig. 2). After baseline measurements, we collected data on SCr and UPCR at 3, 6, and 12 months. Univariate analysis showed that SCr at 12 months was independently associated with urinary β2-MG levels. However, UPCR at 12 months was found to be statistically nonsignificant. These findings illustrate that urinary β2-MG is more significantly associated with ΔSCr than ΔUPCR (Table 3). Additionally, in a multivariate analysis urinary β2-MG was found to be associated only with iSCr and SCr at 12 months (Table 3).
Peters et al. [4] reported urinary excretion of low-molecular-weight proteins did not offer an advantage over total proteinuria and SCr in predicting prognosis in patients with IgAN. However, this study was the first to report the prognostic value of urinary excretion of β2-MG in IgAN. In our study, urinary β2-MG levels showed significant correlations with initial proteinuria and SCr in IgAN. Albumin, β2-MG, elevated iSCr, and UPCR predicted progression of renal disease in univariate Cox analysis (Table 4). Our data indicate that urinary excretion of β2-MG may be an additional prognostic factor in patients with IgAN in addition to traditional risk factors; i.e., elevated SCr, severe proteinuria, and arterial hypertension [3].
In a multiple liner regression analysis, β2-MG showed inverse correlation to Cr after 12 months (standardized β = -1.389, p = 0.046). Conversely, in univariate Cox regression analysis, positive values for standardized β in β2-MG showed conflicting results (standardized β = 0.567, p = 0.041). During follow-up, 45 of 51 patients continuously received treatment after diagnosis. Thus, the inverse correlation to Cr after 12 months will be determined by the impact of treatment (therapeutic group 45/51, 88.24%).
The glomerular grades were significantly related to hypertension, SCr levels, and proteinuria levels at the time of biopsy. For this reason, the glomerular grading system is useful for predicting the natural course of disease in IgAN [5]. The refined H.S. Lee grading system provides important prognostic information and stands as an independent morphological predictor of renal outcome. In this study, the H.S. Lee grading showed no significant correlation with disease progression in IgAN (Table 4).
Interstitial fibrosis results from postinflammatory sclerosis of the interstitium, and the extent of tubulointerstitial lesions are correlated with the deterioration of renal function [10]. However, we found no significant association with disease progression.
Our study had several limitations. First, only a relatively small number of patients were evaluated over a short follow-up period, which may explain the small percentage of patients who showed rapid disease progression and the lack of patients who developed ESRD. Second, this study comprised patients who were mostly treated therapeutically with medications (ACEis and/or ARBs, prednisolone, immunosuppressive agents). To minimize these effects on results, we eliminated the effects of therapy during the data analysis.
In conclusion, urinary β2-MG levels showed significant correlations with iSCr and UPCR in IgAN. Our data indicate that urinary β2-MG may be an additional prognostic factor in patients with IgAN in addition to SCr concentration and urinary protein excretion.
KEY MESSAGE
1. Urinary β2-microglobulin may be an additional prognostic factor in patients with immunoglobulin A nephropathy in addition to serum creatinine concentration and urinary protein excretion.
Notes
No potential conflict of interest relevant to this article was reported.