Clinical features of elderly chronic urticaria
Article information
Abstract
Background/Aims
Chronic urticaria (CU) is defined as itchy wheals lasting 6 weeks or more. As the aged population increases worldwide, it is essential to identify the specific features of this disease in the elderly population.
Methods
We investigated the prevalence and clinical features of CU in elderly patients. Medical records of 837 CU patients from the outpatient Allergy Clinic of Ajou University Hospital, Korea were analyzed retrospectively. Patients with chronic spontaneous urticaria according to the EAACI/GA2LEN/EDF/WAO guidelines were included. Patients older than 60 years were defined as elderly.
Results
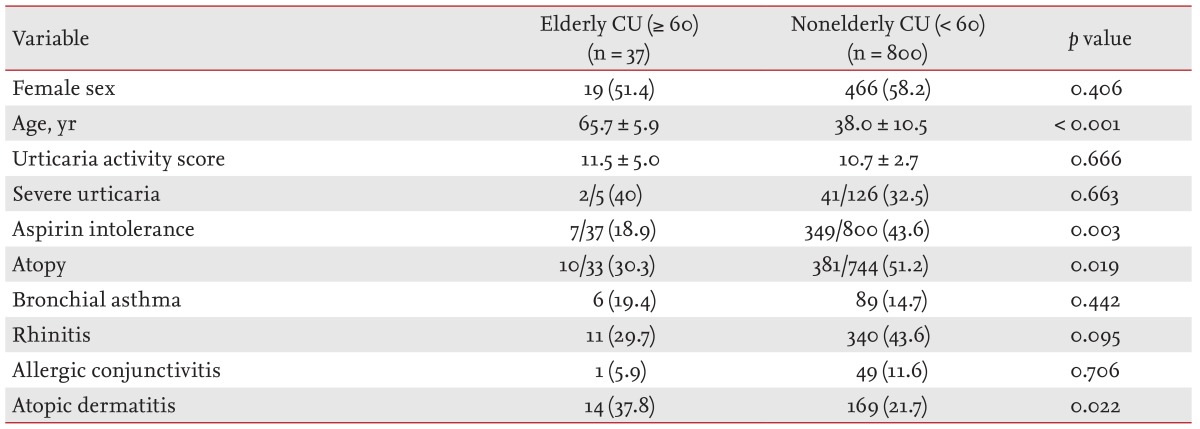
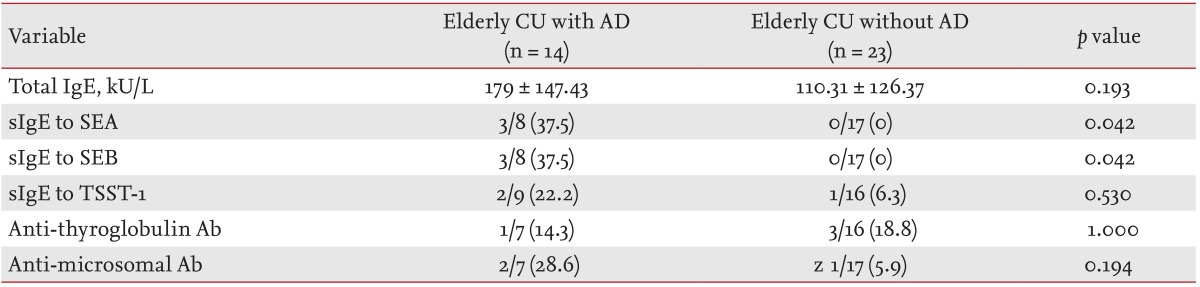
Of the 837 patients, 37 (4.5%) were elderly. In elderly versus nonelderly CU patients, the prevalence of atopic dermatitis (AD) was significantly higher (37.8% vs. 21.7%, respectively; p = 0.022), while that of aspirin intolerance was lower (18.9% vs. 43.6%, respectively; p = 0.003) in terms of comorbid conditions. The prevalences of serum specific immunoglobulin E antibodies to staphylococcal enterotoxin A and staphylococcal enterotoxin B were considerably higher in elderly CU patients with AD than in those without AD (37.5% vs. 0%, respectively).
Conclusions
Elderly patients with CU had a higher prevalence of AD. Therefore, there is a need to recognize the existence of AD in elderly CU patients.
INTRODUCTION
Chronic urticaria (CU) is a common allergic skin disease. It is defined as itchy wheals with or without angioedema that usually persists for less more than 24 hours. According to current EAACI/GA2LEN/EDF/WAO guidelines [1], CU can be classified as spontaneous, physical, or other. Chronic spontaneous urticaria (CSU), usually called CU, is the most common subtype of all forms of nonacute urticaria, and is characterized by wheals that develop independently of external stimuli and last for a minimum of 6 weeks. The underlying causes of CU are difficult to identify in most patients [2].
The pathogenic mechanisms of CU are unclear. Evidence of an autoimmune etiology is reported in about 45% of CU patients, while in others the underlying cause remains unknown. Circulating autoantibodies specific to high-affinity immunoglobulin E (IgE) receptors or dermal mast cell bound IgE activate mast cells and induce degranulation with cytokine release [3]. Atopy is proposed to play a role in the pathogenesis of CU, especially in the aspirin-intolerant CU phenotype [4]. Several hypotheses exist regarding the relationship of atopy and aspirin intolerance; however, the exact association remains unclear [5]. One report has suggested that a personal or familial history of allergic disease is predictive of a response to anti-IgE monoclonal antibody (Omalizumab) therapy. This report also indicated that a personal or familial history of allergic disease might contribute to the development of CU [6].
Superantigens are virulent polypeptides produced by a variety of infectious organisms; those produced by Staphylococcus aureus are particularly important in allergic diseases such as asthma [7], aspirin exacerbated respiratory disease [8], chronic rhinosinusitis [9], atopic dermatitis (AD) [10], and CU [11]. The levels of specific IgE antibodies to superantigens correlate to disease severity in upper and lower airway disease [7,10]. In addition, specific IgE antibodies to staphylococcal superantigens are thought to stimulate mast cells, basophils, and eosinophils, causing release of various mediators [9,12].
The aged population is increasing worldwide. Forty years ago, life expectancy in Korea was 60 years, with only 3% of the Korean population reaching age 65 years or more [13,14]. In 2012, by contrast, those over the age of 65 years represented 12% of the Korean population. It is predicted that the population over age 65 years in Korea will increase to ~40% by 2060 [14]. All organ systems are affected by aging, and geriatric patients respond differently to environmental stimuli. Therefore, it is essential to identify specific features of chronic diseases in aged populations. Several studies have investigated the clinical presentation and etiopathogenesis of urticaria in the elderly population [15,16]; however, few have compared the clinical features of CU in the elderly to those of the nonelderly [17]. We investigated the prevalence and clinical features of CU in elderly versus nonelderly patients in Korea. The findings of this study reveal factors that play a significant role in elderly CU.
METHODS
Subjects
We retrospectively analyzed the medical records of 837 CU patients (age range, 9 to 84 years) who were followed at the outpatient Allergy Clinic of Ajou University Hospital in South Korea. According to EAACI/GA2LEN/EDF/WAO guidelines [1], patients with physical or other secondary types of urticaria were excluded, while those with CSU were included. AD was diagnosed by allergy specialists when patients demonstrated skin itching, dryness, and recurrent typical eczema with a high eosinophil cataionic protein level, along with concomitant allergic diseases or atopy (as defined by a positive skin prick test or high serum-specific IgE antibodies to common inhalant allergens). Subjects were divided into two groups according to the their age at presentation: those younger than 60 years were classified as non-elderly, while those older than 60 years were classified as elderly [18]. This study was approved by the Institutional Review Board of Ajou University Hospital.
Urticaria activity scores and urticaria severity
The urticaria activity score (UAS) was used to assess disease severity. UAS was calculated using the quantity (0, no wheals; 1, < 10 wheals; 2, 10 to 50 wheals; 3, > 50 wheals), distribution range (0, none; 1, < 25% of the body surface area [BSA]; 2, 25% to 50% of BSA; 3, > 50% of BSA), mean diameter (0, no wheals; 1, < 1 cm; 2, 1 to 3 cm; 3, > 3 cm), and duration (0, no wheals; 1, < 4 hours; 2, 4 to 12 hours; 3, > 12 hours) of wheals, along with pruritus according to intensity (0, no pruritus; 1, mild; 2, moderate; 3, severe) within the last week, yielding a total score of 0 to 15 [19]. Severe CU was defined as a UAS ≥13 at the initial visit [20].
Atopic status
Atopy was defined by a positive response to at least one common inhaled allergen, detected by either a skin prick test or serum-specific IgE test. Skin prick tests were performed with 55 common aeroallergens (Bencard, Brentford, UK) and the responses were considered positive if they produced a wheal of more than 3 mm in diameter, or if the ratio of the mean wheal diameter to that of the histamine control was > 1. Specific IgE antibodies were measured using the ImmunoCAP system (Thermo Fisher Scientific, Uppsala, Sweden); a level greater than or equal to 0.35 kU/L was considered a positive response [18].
Immune-related laboratory findings
Total IgE levels and specific IgE antibody levels for staphylococcal enterotoxin A (SEA), staphylococcal enterotoxin B (SEB), and toxic shock syndrome toxin (TSST)-1 were obtained using the ImmunoCAP system (Thermo Fisher Scientific). Anti-thyroglobulin (TG) and anti-microsomal (MC) antibodies were detected by radioimmunoassay (BRAHMS Aktiengesellschaft, Hennigsdorf, Germany).
Oral provocation test with aspirin
An oral provocation test with aspirin was conducted on all study patients [4]. All medications, including anti-histamines, steroids, and leukotriene receptor antagonists, were discontinued 72 hours prior to the procedure. After a placebo challenge, 500 mg of aspirin (Rhonal, KunWha Pharmaceutical Co., Seoul, Korea) in tablet form were administered orally. Patients were observed every 30 minutes for 4 hours following aspirin administration for urticaria, angioedema, or changes in lung function. The appearance of urticaria within 4 hours without any change in forced expiratory volume in 1 second was considered a positive result.
Statistical analysis
Data for continuous variables were compared via Student t tests; Pearson chi-square or Fisher exact tests were used for categorical variables. All computations were performed using the SPSS version 16.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Clinical characteristics and comorbidities
Table 1 summarizes the clinical characteristics of the subjects. Of 837 patients with CU, 37 (4.4%) were elderly. Both the nonelderly and elderly CU groups showed a female predominance (58.2% vs. 51.4%, respectively; p = 0.406). There were no significant differences between the two groups in mean UAS (p = 0.666) or the prevalence of severe CU (32.5% in nonelderly vs. 40% in elderly, p = 0.663). The atopy rate was significantly lower in the elderly CU group than in the non-elderly CU group (30.3% vs. 51.2%, respectively; p = 0.019). The prevalence of AD was considerably higher in the elderly CU group than the non-elderly CU group (37.8% vs. 21.7%, respectively; p = 0.022); however, there were no significant differences in the prevalence of other allergic diseases such as asthma or rhinitis. The prevalence of aspirin intolerance was significantly lower in the elderly CU group than the non-elderly CU group (18.9% vs. 43.6%, respectively; p = 0.003).
Laboratory findings in elderly and nonelderly CU patients
Levels of total IgE and the prevalence of serum-specific IgE antibodies to SEA, SEB, and TSST-1 exhibited no significant differences between the two age groups. No significant differences were noted in the prevalence of serum anti-TG and anti-MC antibodies between the two groups (Table 2).
Table 3 shows laboratory findings according to the coexistence of AD in elderly patients with CU. Elderly CU patients with AD showed no differences in total IgE levels or the prevalence of serum anti-TG or anti-MC antibodies compared to those without AD. The prevalence of serum-specific IgE to SEA/SEB was significantly higher in elderly CU patients with AD compared to those without AD (37.5% vs. 0%, respectively, p = 0.042); however, no significant difference was noted in the prevalence of serum-specific IgE to TSST-1 between the two groups.
DISCUSSION
It is estimated that about 0.5% to 1% of the population suffers from CU at any given time, and that about one quarter of the total population has experienced urticaria at some point during their lives. Both sexes can be affected, but in general, females suffer from urticaria nearly twice as frequently as males [21]. In our study, we found a lack of female predominance in the elderly CU group, despite a 57.9% proportion of females in the total study population. This finding is in concordance with a previous report of an almost equal sex distribution in an elderly CU group [17]. All age groups can be affected by CU, but the peak age is between 20 and 40 years [21]; therefore, few studies on CU in the aged population have been performed. In this study, despite a less stringent definition of elderly as those over 60 years (versus over 65 years used in previous studies), only 4.4% of the subjects were elderly, versus 9.4% in a previous study [17]. Despite the small percentage of CU patients defined as elderly, it is important to identify the specific clinical features of this group due to the aging of the population.
Structural and physiological changes occur in the skin with age. A reduction in dermal thickness and decrease in cutaneous vascularity and cellularity are associated with aging. The number of cutaneous mast cells also declines [22]. The major characteristic of CU pathogenesis is mast cell degranulation, which results in release of histamine and other chemical mediators. Cutaneous mast cell numbers are not increased in CU; however, an increased releasability of histamine (related to the histamine content or activation state of mast cells) may play a role in CU pathogenesis [23]. The features of elderly CU are not due to structural and physiological changes in aging skin; therefore, our results showing no significant differences between the two groups in UAS or in the prevalence of severe CU exist despite changes in dermal thickness and the number of mast cells. This similarity in UAS for both groups is in agreement with a previous report [17]. To estimate urticarial activity accurately, recent trials have used UAS 7, which averages the daily UAS over 1 week [24]. Because of the limitations of the UAS 7 in real practice, we applied a UAS including five components, as described previously [19,20]. Therefore, we speculate that CU disease activity and severity are not significantly different between the elderly and nonelderly.
Superantigens (derived mainly from S. aureus) can activate T cells, induce IgE synthesis by B cells, and stimulate inflammatory leukocytes (such as mast cells and eosinophils). CU patients were reported to have high levels of serum-specific IgE against SEA, SEB, and TSST-1 [25]. The prevalence of specific IgE antibodies against SEA, SEB, and TSST-1 were noted to be significantly higher in aspirin intolerant chronic urticaria (AICU) patients than in normal controls. Compared to aspirin tolerant chronic urticaria patients, levels of serum specific IgE to TSST-1 were significantly higher in AICU patients [12]. With regard to AD, one report described higher levels of specific IgE antibodies to staphylococcal superantigens in younger subjects [26]. However, another study found that 57% of adult AD patients had high levels of serum-specific IgE to staphylococcal superantigens, compared to only 34% of pediatric AD patients [27]. To our knowledge, no study has investigated differences in the prevalence of specific IgE antibodies to staphylococcal superantigens according to age within the adult population, especially in the case of CU. We identified no difference in the prevalence of specific IgE to staphylococcal superantigens between the elderly and nonelderly CU groups; however, the prevalences of specific IgE antibodies to SEA and SEB were significantly higher in elderly CU patients with AD than in those without. The rate of AD in the general population is 2% to 5% (~15% in children and young adults), and is believed to be lower in the elderly than young adults (~5.6%) [27,28]. Studies of the prevalence of AD accompanied by CU in the elderly are limited; however, one population-based cross-sectional survey reported higher rates of eczema and urticaria in adults [28]. The higher prevalence of AD in elderly CU patients in our study may be due to presumptive diagnosis of AD based on clinical signs [17]. In patients with AD, impaired skin barrier function facilitates the colonization of S. aureus; ceramidases from this organism degrade ceramide (a component in the lipid matrix of the skin barrier). By this mechanism, S. aureus enables continuous penetration of the skin barrier, leading to IgE sensitization [29]. Even normal-appearing aged skin has increased ceramidase activity, resulting in degradation of the lamellar structure of stratum corneum lipids [30]. Therefore, production of specific IgE antibodies to staphylococcal superantigens may play a synergistic role in the pathogenesis of elderly AD.
Aspirin intolerance and atopy were found more frequently in the nonelderly than the elderly CU group; however, it has already been noted that a personal or family history of atopy is a risk factor for aspirin intolerance in CU patients [5]. Most studies indicate a lower prevalence of atopy in the elderly population [31]; therefore, we tested a bivariate predictive model of nonelderly CU. The multiple logistic regression analysis included aspirin intolerance and atopy variables. However, aspirin intolerance was significantly related to age (p = 0.031), even after adjustment for the confounding effect of atopic status. It is widely known that aspirin aggravates CU in 20% to 30% of patients [32]. However, 42.5% of CU patients in our study were found to have aspirin intolerance, a considerably larger proportion than reported previously [33,34]. This is most likely due to recruitment of study subjects from a tertiary care university hospital. Nevertheless, the high prevalence of aspirin intolerance in nonelderly CU patients indicates a need to carefully question and educate CU patients about the possibility of aspirin allergy.
In conclusion, this study indicates that 4.5% of CU patients are elderly. There is a need to further investigate the relationship of AD and CU in elderly patients, due to the higher prevalence of AD in elderly versus nonelderly CU patients.
KEY MESSAGE
Clinical features of elderly chronic urticaria (CU) patients were different from those in younger population.
Atopic dermatitis is a common comorbid condition of elderly CU.
Evaluation of comorbid condition is needed for the management of elderly CU.
Acknowledgments
This research was supported by a grant of Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI14C006).
Notes
No potential conflict of interest relevant to this article was reported.