Early effects of tumor necrosis factor inhibition on bone homeostasis after soluble tumor necrosis factor receptor use
Article information
Abstract
Background/Aims
Our aim was to assess whether short-term treatment with soluble tumor necrosis factor (TNF) receptor affects circulating markers of bone metabolism in rheumatoid arthritis (RA) patients.
Methods
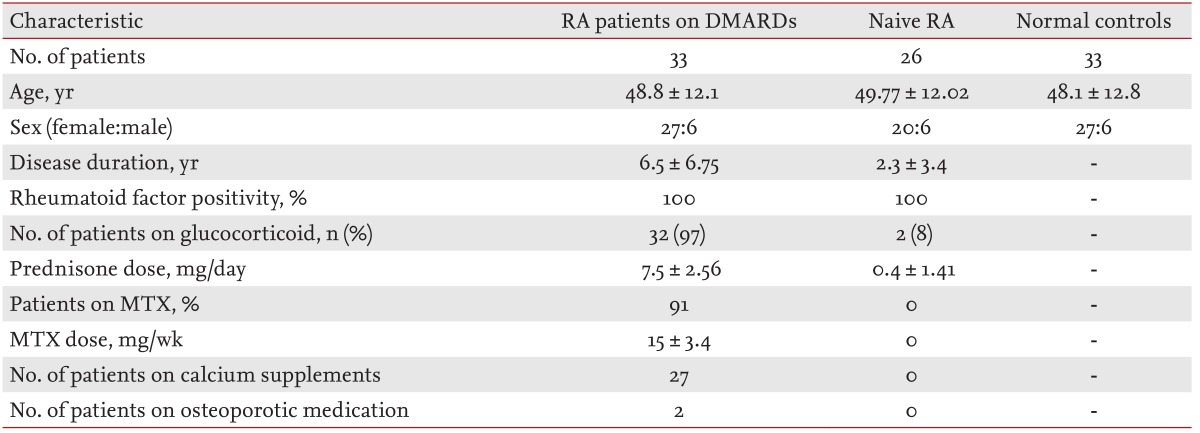
Thirty-three active RA patients, treated with oral disease-modifying antirheumatic drugs (DMARDs) and glucocorticoids for > 6 months, were administered etanercept for 12 weeks. Serum levels of bone metabolism markers were compared among patients treated with DMARDs at baseline and after etanercept treatment, normal controls and naive RA patients not previously treated with DMARDs (both age- and gender-matched).
Results
Bone-specific alkaline phosphatase (BSALP) and serum c-telopeptide (CTX)-1 levels were lower in RA patients treated with DMARDs than in DMARD-naive RA patients. After 12 weeks of etanercept treatment, serum CTX-1 and sclerostin levels increased. In patients whose DAS28 improved, the sclerostin level increased from 1.67 ± 2.12 pg/mL at baseline to 2.51 ± 3.03 pg/mL, which was statistically significant (p = 0.021). Increases in sclerostin levels after etanercept treatment were positively correlated with those of serum CTX-1 (r = 0.775), as were those of BSALP (r = 0.755).
Conclusions
RA patients treated with DMARDs showed depressed bone metabolism compared to naive RA patients. Increases in serum CTX-1 and sclerostin levels after short-term etanercept treatment suggest reconstitution of bone metabolism homeostasis.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic disease characterized by joint inflammation and local bone erosion. Progressive bone erosion and joint destruction results in progressive joint deformity, which is the hallmark of RA [1]. Tumor necrosis factor (TNF)-α is a key player in the pathogenesis of RA, and is associated with both inflammation and bone metabolism. TNF-α induces the secretion of multiple proinflammatory cytokines (e.g., interleukin [IL]-1, IL-6, IL-8, and granulocyte macrophage colony-stimulating factor), as well as dickkopf (DKK)-1, a regulator in the Wnt pathway, which is associated with bone formation. Blockade of TNF-α is known to arrest bone loss [2,3], partly by stimulating bone formation [4]. Diarra et al. [4] showed that treatment with an anti-TNF blocker decreases circulating DKK-1 levels in RA patients, and a previous study suggested DKK-1 levels are associated with radiographic progression in RA patients [5]. Therefore, the role of the Wnt pathway, especially regulatory molecules such as DKK-1 and sclerostin, is a recent focus of RA research. However, data on the effects of in vivo TNF-α inhibition on general bone loss in RA patients are limited [2,6,7]. Furthermore, the effects of etanercept, a soluble TNF receptor, on Wnt pathway antagonists have not been determined. Therefore, our aim was to assess whether short-term treatment with a soluble TNF receptor in RA patients affects circulating markers of bone metabolism, including sclerostin.
METHODS
Patients
We enrolled 33 RA patients who had been treated with disease-modifying antirheumatic drugs (DMARDs) and glucocorticoids for > 6 months. Twelve patients were postmenopausal females, 15 were premenopausal females, and six were males. All patients fulfilled at least four of the 1987 revised American College of Rheumatology criteria for RA [8]. All patients had active RA with a 28 joint count disease activity score (DAS28) > 3.2 at inclusion [9], despite chronic use of DMARDs and glucocorticoids. Thirty of the 33 patients received combination DMARD treatment including methotrexate (MTX) at a dose of > 12.5 mg/week; DMARDs other than MTX were discontinued after starting etanercept therapy. The three patients who did not receive MTX prior to the study due to contraindications were treated with combination DMARDs, including hydroxychloroquine, azathioprine, sulfasalazine, and bucillamine; either hydroxychloroquine or azathioprine was continued with etanercept throughout the study period. Twenty-seven patients were treated with calcium and vitamin D supplements and two patients were taking osteoporosis medications (raloxifene and risedronate for each). All patients in this group were injected with etanercept at 25 mg twice per week for 12 weeks.
An equal number of age- and gender-matched healthy individuals who visited Inha University Hospital for health screening were recruited as normal controls. RA patients who were just diagnosed as seropositive RA and were not yet receiving any DMARD treatment were also included as naive RA.
Blood samples were collected from RA patients previously treated with DMARDs at baseline and after 12 weeks of etanercept treatment, normal controls, and naive RA patients. The demographic characteristics of patients are summarized in Table 1. This study was approved by the Ethics Committee of Inha University Hospital (No. 2010-0001) in January 2010 and informed consent was obtained from all participants.
Clinical assessments
Overall disease activity was evaluated by the DAS28, which includes three variables of 28 joint counts of tenderness and swelling and erythrocyte sedimentation rate (ESR). DAS28 was calculated using a calculator (online at www.das-score.nl). Acute-phase reactants, including ESR and C-reactive protein (CRP), were measured before and after 12 weeks of etanercept treatment. The European League Against Rheumatism (EULAR) response criteria were used to assess response to therapy after 12 weeks of treatment [9].
Biochemical analysis
Serum samples were stored immediately at -70℃ until analysis. Serum levels of IL-6, receptor activator of nuclear factor-kappaB ligand (RANKL), osteoprotegerin (OPG), c-telopeptide (CTX)-1, sclerostin, bone-specific alkaline phosphatase (BSALP), DKK-1, and procollagen type 1 amino-terminal propeptide (P1NP) were measured using commercially available enzyme-linked immunosorbent assay kits (IL-6, R&D Systems, Minneapolis, MN, USA: interassay coefficient of variation [CV] 6.9% to 7.8%; RANKL and OPG, Biomedica, Wien, Austria: interassay CV of RANKL 3% to 5%, interassay CV of OPG 4% to 10%; CTX-1, Nordic Bioscience Diagnostics A/S, Herlev, Denmark: interassay CV 2.5% to 10.9%; sclerostin, USCN, Wuhan, China: interassay CV 4% to 6%; BSALP, Quidel, San Diego, CA, USA: interassay CV 5% to 8%; DKK-1, Assay Designs, Michigan, USA: interassay CV 7.9% to 13.3%; P1NP, Cusabio, Wuhan, China).
Bone mineral density measurement
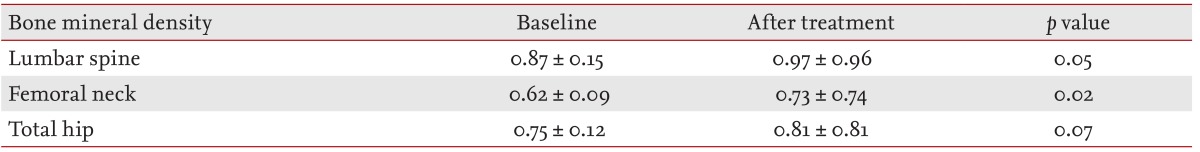
The bone mineral density (BMD) in RA patients was measured by dual-energy X-ray absorptiometry (QDR-4500A, Hologic Inc., Bedford, MA, USA). BMD was measured at baseline and 1 year after treatment in the lumbar spine, femoral neck, and total hip.
Statistical analysis
Data are presented as means ± standard deviation. Results were analyzed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). Data prior to and after etanercept treatment were compared using the paired samples t test. Comparisons between two independent groups were performed by independent samples t test. Correlations between parameters were assessed using Pearson's test. A value of p ≤ 0.05 was regarded as significant in all analyses.
RESULTS
RA patients previously treated with DMARDs received etanercept treatment for 12 weeks and reported no side effects throughout the study period.
Effects of etanercept on systemic inflammation and RA disease activity
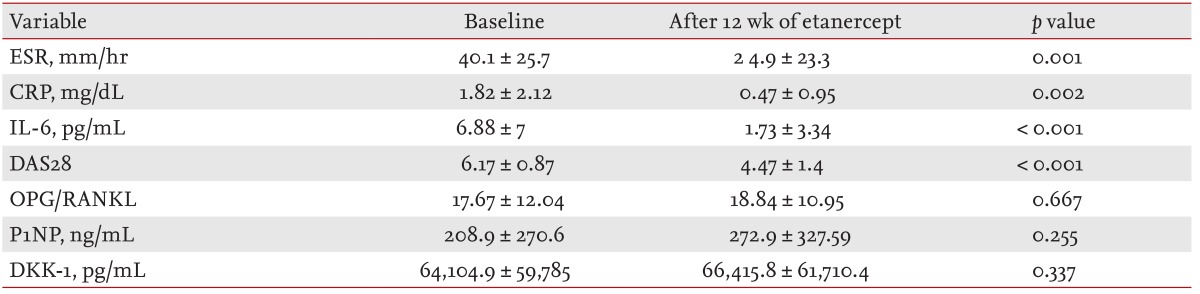
Etanercept treatment caused notable reductions in markers of systemic inflammation, including ESR and CRP (Table 2). The serum IL-6 level also declined significantly upon treatment (p < 0.001). RA disease activity was also improved (p < 0.001).
Effects of etanercept on bone metabolism
In RA patients treated chronically with DMARDs and glucocorticoids prior to etanercept, both BSALP and CTX-1 levels were significantly lower than in RA patients not yet treated with any DMARD or glucocorticoids (p = 0.004, p = 0.024, respectively). After 12 weeks of etanercept treatment, serum BSALP, sclerostin, and CTX-1 levels increased, the latter significantly (Fig. 1). Increases in sclerostin levels after etanercept treatment correlated positively with those of serum CTX-1 (r = 0.775, p < 0.001). Examination of serum levels of bone metabolism markers revealed that changes in BSALP were correlated with those in CTX-1 (r = 0.755, p < 0.001) (Fig. 2). Levels of other bone metabolism markers including, OPG/RANKL, P1NP, DKK-1, were not affected significantly by etanercept treatment (Table 2).

(A) Serum c-telopeptide (CTX)-1, (B) sclerostin, and (C) bone-specific alkaline phosphatase (BSALP) levels among normal controls, drug-naive rheumatoid arthritis (RA) patients, and RA patients treated chronically with disease-modifying antirheumatic drugs (DMARDs) at baseline and after 12 weeks of etanercept treatment.
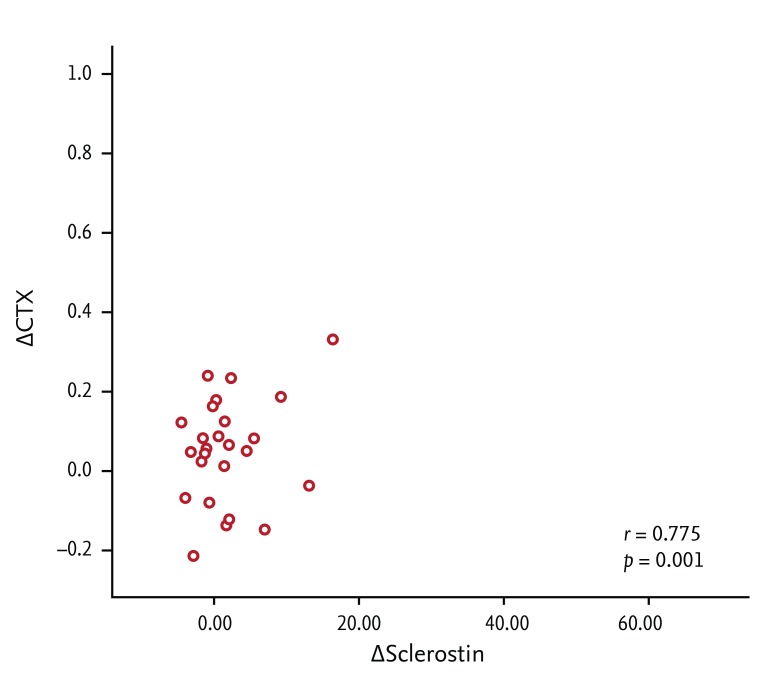
Correlation between etanercept-induced changes in serum sclerostin and c-telopeptide (CTX)-1 levels (r = 0.775, p < 0.001).
By the DAS28 EULAR response criteria, there were 27 responders and six nonresponders. In the responders, of the bone metabolism markers only serum sclerostin levels increased significantly, from 1.67 ± 2.12 to 2.51 ± 3.03 pg/mL (p = 0.021). Bone metabolism marker levels did not change in the nonresponder group.
Lumbar spinal and femoral neck BMD (g/cm2) greatly improved at approximately 1 year of treatment with etanercept (p = 0.050, p = 0.021, respectively). Total hip BMD also exhibited a trend towards recovery after treatment (p = 0.066) (Table 3).
DISCUSSION
The main finding of this study was that serum levels of the bone metabolism markers CTX-1 and BSALP were lower in RA patients treated chronically with DMARDs and glucocorticoids than in DMARD-naive RA patients. Etanercept treatment for 12 weeks improved RA disease activity and systemic inflammation, but serum levels of the bone metabolism markers CTX-1 and sclerostin remained elevated after treatment.
Our finding that chronic treatment with DMARDs and glucocorticoids reduces serum CTX-1 and BSALP levels is consistent with earlier reports that these drugs affect bone formation and resorption. MTX, which was taken by 91% of the RA patients in this study treated chronically with DMARDs, suppresses bone formation by inhibiting the differentiation of early osteoblastic cells [10]. MTX is also known to decrease deoxypyridinoline (DPD) and N-telopeptide of type 1 collagen levels in patients with RA, thereby ameliorating bone resorption [11]. Glucocorticoids, taken by 97% of the patients chronically treated with DMARDs, also affect bone formation and resorption. Chronic prednisolone at 5 mg daily for 8 weeks was found in a double-blinded randomized trial to significantly decrease serum P1NP, osteocalcin, and free urinary DPD levels [12].
Our finding of increased serum CTX-1 and sclerostin after 12 weeks of etanercept treatment has several implications for managing RA patients' bone density and disease. Serum CTX-1 is known to be more specific to bone resorption than other measurements, as these peptide fragments are generated by collagen degradation [13]. In contrast, sclerostin is a secreted Wnt antagonist that binds to low density lipoprotein receptor-related protein 5 (LRP5) and LRP6 to inhibit Wnt/β-catenin signaling [14,15,16], suggesting that it would inhibit bone formation [17].
The present finding that etanercept stimulates bone resorption and increases serum levels of a bone formation antagonist is inconsistent with previous studies showing improved bone metabolism after 12 weeks of etanercept therapy [7,18]. However, whether this indicates worsening of bone metabolism after short-term etanercept therapy is unclear. Polyzos et al. [19] showed that risedronate for 6 months in postmenopausal females significantly increased sclerostin levels; further, sclerostin levels were positively correlated with lumbar spinal BMD. Similarly, Terpos et al. [20] found that tocilizumab, an IL-6 blocker, increases the sclerostin level and increases the ratio of OPG to RANKL after 2 months of treatment. Thus, increases in sclerostin after etanercept use suggest not only decreased bone formation but also a therapeutic effect on RA-induced bone degradation. In addition, Polyzos et al. [19] reported that levels of bone metabolism markers such as CTX, P1NP, and total serum ALP were increased after 6 months of teriparatide treatment. In our study, the OPG/RANKL ratio, P1NP and BSALP were not altered significantly by etanercept therapy. However, its effects on serum CTX-1 and sclerostin and the correlation of serum CTX-1 with BSALP suggest that bone resorption and formation might be coupled and that etanercept might induce recovery of bone metabolism from DMARD- and glucocorticoid-induced depression.
The correlation of changes in sclerostin with changes in serum CTX-1 are consistent with the study of Modder et al. [21], who reported the same correlation after estrogen treatment. Although the underlying mechanism is unknown, sclerostin seems to affect bone resorption in addition to bone formation [21].
The fact that increases in the sclerostin level after etanercept treatment were significant only in DAS28 responders suggests that the effects of etanercept on bone metabolism might result from the reduction in systemic inflammation. Similarly, Sennels et al. [22] reported a significant increase in total serum RANKL in tandem with major decreases in serum CRP after 12 weeks of etanercept.
The main limitation of our study was that it was not randomized. Secondly, although comparisons between baseline and 12 weeks after etanercept therapy were performed in individual patients, comparisons between drug-naive patients and those treated chronically with DMARDs were conducted across groups. Though a longitudinal study would reduce the probability of individual variation, Korean treatment guidelines mean that such a study would take years; nonresponse after at least 6 months of oral DMARDs is required for treatment with a TNF inhibitor at insurance-covered costs. Individual variations such as differences in sex hormone levels could also have affected our results.
In conclusion, 12 weeks of etanercept therapy appears to improve systemic inflammation, but not bone turnover. However, it may begin to restore bone metabolism at this time point, which is depressed due in part to chronic use of MTX and glucocorticoids.
KEY MESSAGE
Twelve weeks of etanercept treatment dramatically reduces systemic inflammation.
Levels of circulating bone metabolism markers-including c-telopeptide-1 and sclerostin-are elevated after etanercept treatment.
Short-term etanercept therapy appears to restore homeostasis in bone metabolism following chronic use of methotrexate and glucocorticoids.
Acknowledgments
We thank all of our patients and volunteers who donated blood for this study. This study was supported by an Inha University Research Grant (grant number 42159-01).
Notes
No potential conflict of interest relevant to this article was reported.