Utilization of the respiratory virus multiplex reverse transcription-polymerase chain reaction test for adult patients at a Korean tertiary care center
Article information
Abstract
Background/Aims
Respiratory viruses (RVs) are considered to be important respiratory pathogens in adult patients, and the multiplex reverse transcription-polymerase chain reaction (RT-PCR) test is used frequently in adult patients with respiratory infections. However, clinical data regarding utilization of the multiplex RT-PCR test for RVs are lacking.
Methods
We investigated the utilization of the multiplex RT-PCR test for RVs at Chung-Ang University Hospital in Seoul, Korea, between January 2012 and April 2013.
Results
During the study period, the multiplex RT-PCR test was performed for 291 adult patients. The test frequency was 4.9% of rapid influenza antigen detection tests and 0.8% of respiratory bacterial culture studies. A turnaround time of < 48 hours was observed in 25.9% of positive tests. Most of the tests were performed for admitted patients (97.9%) with a community-acquired infection (84.2%) during the flu season (82.5%). RVs were detected in 81 of 291 cases (27.8%). The RV positivity rates for community- and hospital-acquired infections did not differ (28.6% vs. 23.9%, p = 0.52). Of 166 patients with pneumonia, 44 (26.5%) had a viral infection. Among the patients with RV-associated pneumonia, an RV other than influenza was detected in 20 patients (45.4%).
Conclusions
The multiplex RT-PCR test for RVs was infrequently performed at a tertiary care center, and the test results were often reported late. The test was most often performed for admitted adult patients with community-acquired infections during the flu season. The utilization of multiplex RT-PCR testing for RVs in current clinical practice should be improved.
INTRODUCTION
In pediatric patients, respiratory viruses (RVs) are considered important pathogens in acute respiratory tract infections, resulting in hospitalization and acute care visits [1,2,3] for which diagnostic evaluations for RVs are frequently performed [4]. However, diagnostic evaluations for RVs are rarely performed in adult patients because such viruses cause only benign respiratory tract infections [4]. Recently, with the advent of more sensitive molecular techniques, clinical cases of pneumonia associated with variable RVs have been reported with increasing frequency in adult patients [5,6]. The importance of RVs in adult patients has also been emphasized with the emergence of severe acute respiratory syndrome, avian influenza A (H5N1), and pandemic influenza H1N1 in 2009 [3]. In the near future, RV detection tests may be considered an essential part of the diagnostic work-up for adult patients with acute respiratory tract infections. However, physicians treating adult patients are not yet familiar with RV detection tests. We investigated the current utilization of a multiplex reverse transcription-polymerase chain reaction (RT-PCR) test for RVs in adult patients in clinical practice. The multiplex RT-PCR test allows the detection of a large number of RVs simultaneously with a higher sensitivity than viral culture [7]. We hypothesized that utilization data and results from current multiplex RT-PCR tests would be helpful in identifying the benefits and problems associated with multiplex RT-PCR utilization in adult patients.
METHODS
Patient selection and data collection
This study was performed at Chung-Ang University Hospital, an 850-bed, tertiary care teaching hospital in Seoul, Republic of Korea. Adult patients (> 16 years of age) who underwent multiplex RT-PCR testing between January 2012 and April 2013 were identified and their electronic medical records and chest radiographs reviewed. Demographic characteristics, underlying diseases, multiplex RT-PCR results, the presence of respiratory symptoms, and clinical outcomes were investigated.
Definitions
An upper respiratory infection (URI) was defined as the presence of ≥ 1 of the following respiratory symptoms: cough, sputum production, rhinorrhea, sore throat, and dyspnea. Pneumonia was defined as the presence of a new or progressive infiltrate on chest radiography plus two or more of the following symptoms or signs: fever, sputum production, rhinorrhea, sore throat, dyspnea, and the attending physician's diagnosis of pneumonia. A nonrespiratory infection (NRI) was defined as neither a URI nor pneumonia. If a patient had an episode of acute infection within 2 days after admission and underwent multiplex RT-PCR testing for the episode, he or she was considered to have received a test for a community-acquired infection. The flu season was defined as between January and April in 2012 and between January and April in 2013. The flu season was determined based on the Weekly Surveillance Reports for Influenza and Other Respiratory Viruses of the Korea Centers for Disease Control and Prevention [8].
Specimens
During the study period, nasopharyngeal specimens obtained using a flocked swab were submitted in Universal Transport Medium (COPAN, Brescia, Italy). Nasopharyngeal specimens were submitted for RV7 detection between January 2012 and December 2012 and for RV16 detection between January 2013 and April 2013.
Nucleic acid extraction and reverse transcription
Nucleic acids were extracted from 300-µL specimens using a Viral Gene-Spin Viral DNA/RNA Extraction Kit (iNtRON Biotechnology, Seongnam, Korea). cDNAs were synthesized from the extracted RNAs with cDNA Synthesis Premix (Seegene, Seoul, Korea) and a GeneAmp PCR System 9700 thermal cycler (Applied Biomaterials, Foster City, CA, USA).
RV7 testing
RV7 testing was performed to detect the following viruses: adenovirus, influenza viruses A and B, respiratory syncytial virus (RSV), human metapneumovirus (HMPV), and human rhinovirus (HRV) A. PCR was performed using a Seeplex RV7 Detection Kit (Seegene) according to the manufacturer's instructions with a GeneAmp PCR System 9700 thermal cycler (Applied Biomaterials). The products were separated on 2% agarose gels containing 0.5 g of ethidium bromide/mL in Tri-borate-EDTA buffer and were visualized under ultraviolet light.
RV16 testing
RV16 testing was performed to detect the following viruses: adenovirus, influenza viruses A and B, RSV A, RSV B, parainfluenza viruses 1 to 4, HRV, HMPV, human enterovirus, coronavirus 229E, coronavirus NL63, coronavirus OC43, and human bocavirus. During the RV16 test, an internal control was added to each specimen to check the entire process from nucleic acid extraction to PCR, according to the manufacturer's instructions. An Anyplex II RV16 Detection Kit (Seegene) was used to detect fourteen types of RNA viruses and two types of DNA viruses, according to the manufacturer's instructions. Briefly, the assay was conducted in a final volume of 20 µL containing 8 µL of cDNA, 4 µL of 5 × RV primer, 4 µL of 8-methoxypsoralen solution, and 4 µL of 5 × master mix with the CFX96 real-time PCR detection system (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Statistical analyses
Statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were compared using Student t test or the Mann-Whitney U test. Categorical variables were compared using a chi-square test or Fisher exact test.
RESULTS
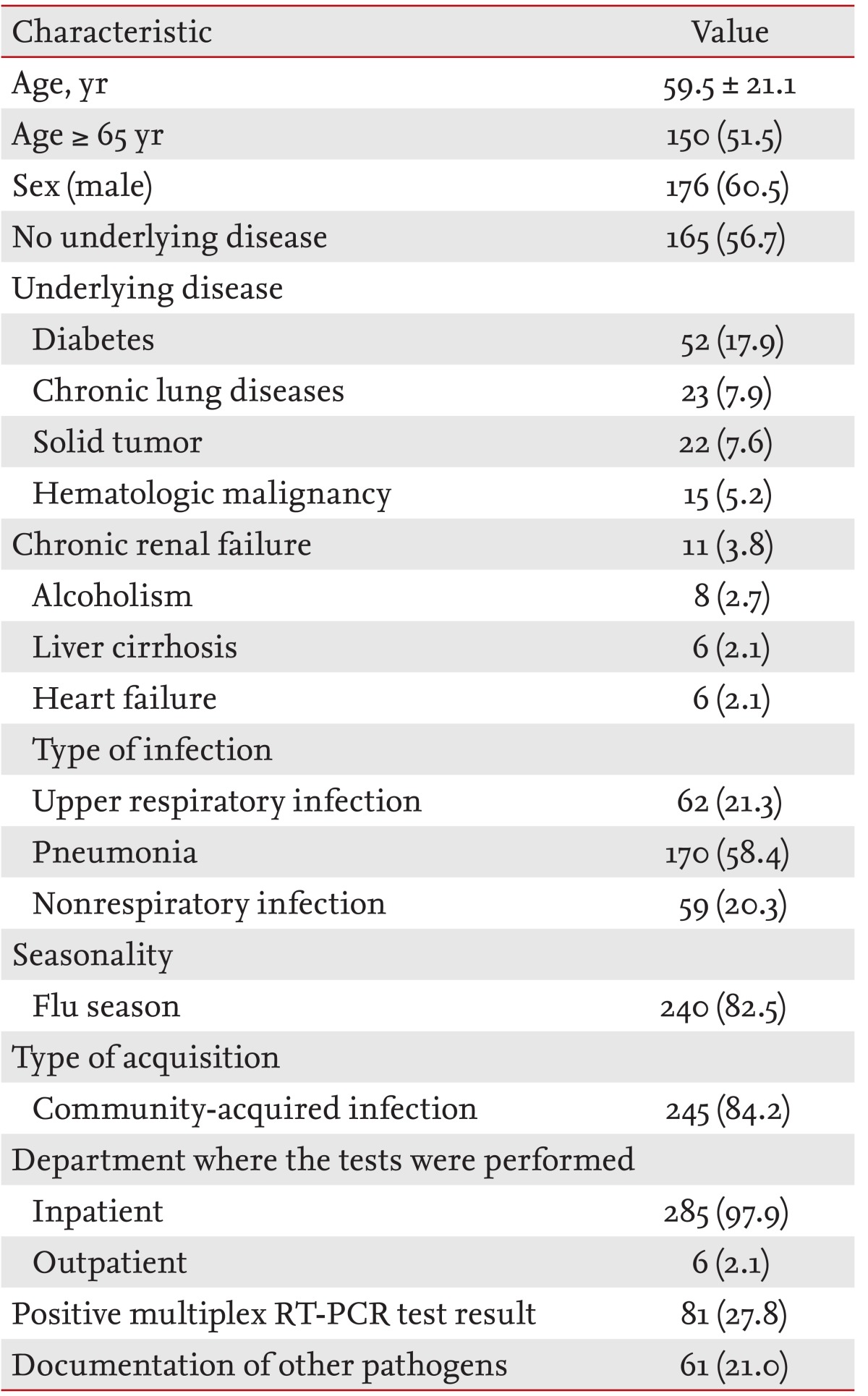
During the study period, multiplex RT-PCR testing was performed for 291 respiratory samples from 282 adult patients. During the same period, rapid influenza antigen detection tests were performed for 5,890 nasopharyngeal swab samples and bacterial cultures were performed for 38,195 respiratory samples in the study hospital. Thus, the frequency of multiplex RT-PCR testing was only 4.9% of rapid influenza antigen detection tests and 0.8% of respiratory bacterial cultures. The mean turnaround time for the 81 positive multiplex RT-PCR tests was 66.1 hours (SD, 24.2; range, 20.4 to 119.7). A turnaround time of < 48 hours was observed in 25.9% (21/81) of the 81 positive tests. The characteristics of the 291 cases are summarized in Table 1. The mean patient age was 59.5 years and more than half were male (176, 60.5%). The most common underlying disease was diabetes mellitus (17.9%), followed by chronic lung disease (7.9%), solid tumor (7.6%), hematologic malignancy (5.2%), and chronic renal failure (3.8%). Multiplex RT-PCR testing was performed most frequently in cases of pneumonia (58.4%), followed by URI (21.3%) and NRI (20.3%). The majority of multiplex RT-PCR tests were performed for admitted patients (97.9%) with community-acquired infections (84.2%) during the flu season (82.5%). Patients who underwent multiplex RT-PCR for an NRI had the following diseases: urinary tract infections, acute gastroenteritis, acute hepatitis, febrile neutropenia, meningitis, mediastinitis, pulmonary tuberculosis, mumps, chickenpox, acute cholangitis, Escherichia coli bacteremia of unknown origin, enteric fever, Clostridium difficile infection, acute appendicitis, cellulitis, cervical lymphadenitis, hemorrhagic cystitis, cerebrovascular accidents, seizure, hyperventilation syndrome, and angina. In patients with an NRI, multiplex RT-PCR testing was performed due to the presence of a fever of unknown origin during the flu or nonflu season.
Characteristics of 291 cases who underwent an respiratory virus multiplex reverse transcription-polymerase chain reaction test (n = 291)
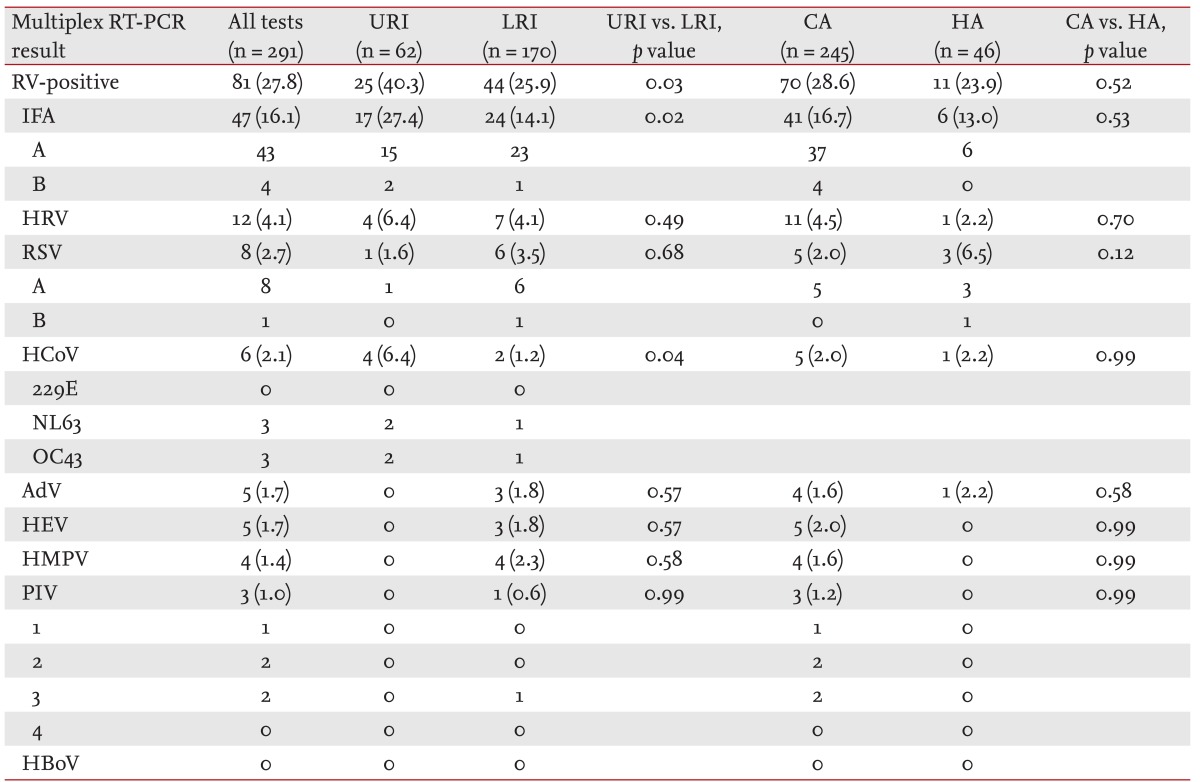
The list of detected RVs is presented in Table 2. RVs were identified in 81 of 291 samples (27.8%), with influenza as the most commonly identified (47/81, 58.0%). Influenza (27.4% vs. 14.1%, p = 0.02) and human coronavirus (6.4% vs. 1.2%, p = 0.04) were more frequently detected in patients with a URI than in those with pneumonia. The rates of RV positivity were not different between patients with community- and hospital-acquired infections (28.6% vs. 23.9%, p = 0.52). For any individual RV, there was no difference between the rates of RV positivity in community- and hospital-acquired infections. Of 59 patients with an NRI, 12 (20.3%) had positive multiplex RT-PCR results for influenza (6), adenovirus (2), parainfluenza virus (2), human enterovirus (2), HRV (1), and RSV (1).
Respiratory viruses detected from 291 respiratory samples using multiplex reverse transcription-polymerase chain reaction testing
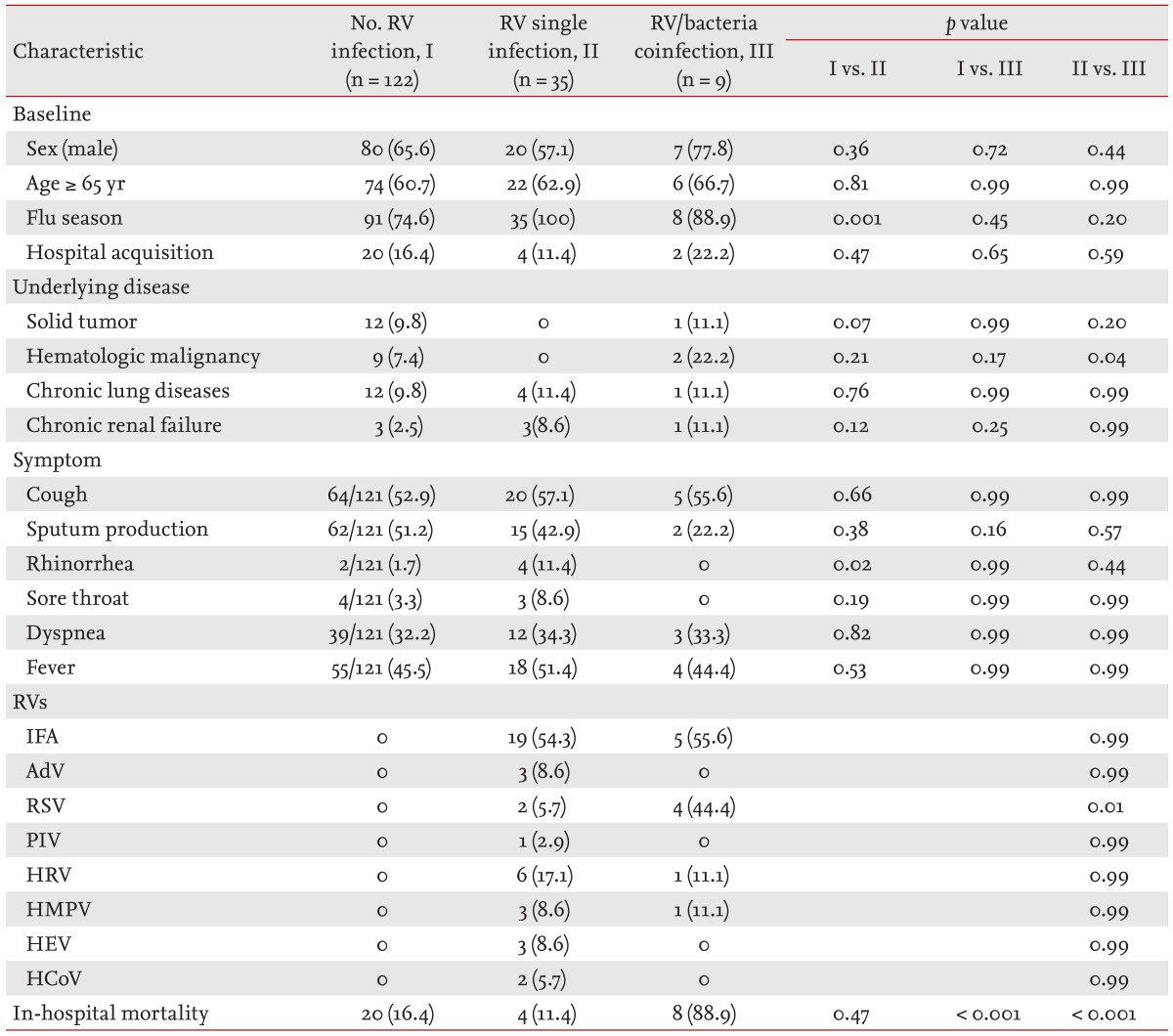
The characteristics of the 166 patients with pneumonia (four duplicates excluded) who underwent multiplex RT-PCR testing were compared according to pathogen type (Table 3). Approximately one quarter of these patients had an RV infection (26.5%, 44/166) and a bacterial infection (24.1%, 40/166), respectively. Nine patients (5.4%, 9/166) had bacterial/viral coinfections. The remaining 91 patients (54.8%) had no identified pathogen. In patients with an RV infection, 20 had RVs other than influenza (20/44, 45.4%). Patients with a single RV infection and those without an RV infection did not differ in terms of their baseline characteristics, underlying diseases, symptoms, and in-hospital mortality, except that rhinorrhea was more frequently observed in patients with a single RV infection during the flu season. In patients coinfected with an RV and a bacterium, RSV and hematologic malignancy were more common features than in patients infected with a single RV. The in-hospital mortality rates were higher in patients with a bacterial/viral coinfection (88.9%) than in those without an RV infection (16.4%) and with a single RV infection (11.4%).
DISCUSSION
Multiplex RT-PCR testing was performed primarily for adult patients with community-acquired respiratory infections admitted to a tertiary care center during the flu season, and especially for patients with pneumonia. The test was performed infrequently and the test results were often reported late. The RV-positive rate (28.6%) for community-acquired infections was not different from that for hospital-acquired infections (23.9%, p = 0.52). RVs were identified in more than a quarter (26.5%) of the 166 patients with pneumonia. Nearly half of the patients with RV-associated pneumonia had RVs other than influenza (20/44, 45.4%).
In our hospital, multiplex RT-PCR testing was most frequently performed for adult patients with community-acquired respiratory infections admitted during the flu season. Thus, the test was primarily used to diagnose influenza in adult patients, especially those with pneumonia. For clinicians who experienced the influenza A (H1N1) pandemic in 2009, the use of multiplex RT-PCR as a diagnostic test for influenza infection is understandable. At other hospitals, the perception of multiplex RT-PCR testing by physicians may be similar, given the predominance of influenza among RVs and the presence of effective anti-influenza therapies [9]. Therefore, the multiplex RT-PCR test should be performed to diagnose influenza during the flu season, especially considering the low sensitivity of rapid influenza antigen detection tests [9]. However, in our hospital, the multiplex RT-PCR test was performed infrequently compared to rapid influenza antigen detection tests. Additionally, the test was rarely performed in outpatient departments at the study hospital. These findings are understandable given that the test results are not available on-site, unlike the rapid influenza antigen detection test. Moreover, the test is not covered by National Health Insurance of South Korea and is relatively expensive (more than 100,000 KRW) compared to the cost of a respiratory bacterial culture (21,619 KRW) or the rapid influenza antigen detection test (18,000 to 28,000 KRW). Consequently, infrequent ordering resulted in less routine PCR testing in the clinical laboratory (not every day, but three times a week at our hospital). This may explain why a turnaround time of < 48 hours was observed in only 25.9% of the positive tests. Forty-eight hours is the most appropriate time period for the introduction of anti-influenza drugs after the onset of symptoms [9]. Considering these problems and the continuous threat of influenza infection, our current utilization of multiplex RT-PCR testing requires improvement, which may increase the diagnostic sensitivity for influenza and decrease the number of patients who receive delayed therapy.
In our study, the RV positivity rate for community-acquired infections did not differ from that for hospital-acquired infections. There were no differences in viral pathogens between community- and hospital-acquired infections. In a recent study of viral infections in patients with severe pneumonia requiring intensive care unit admission, patients with community-acquired pneumonia and healthcare-associated pneumonia had similar rates of RV positivity (40.6% vs. 34.3%) and similar RV pathogens [6]. Regarding these findings, experts suggest that RV infections are not directly influenced by previous healthcare interventions or exposure to antimicrobial agents, but rather they mirror circulating viruses in the community [10]. Although hospital-acquired infections and healthcare-associated infections are different, a similar explanation may be applied to our findings. Considering the frequent detection of RVs in our patients with a hospital-acquired infection, multiplex RT-PCR testing should not be limited to patients with community-acquired respiratory infections. The test may be required for patients with serious hospital-acquired respiratory infections of unknown cause. Based on our study findings, the test may be needed for hospital RV infection control [4], although the turnaround time may not be optimal for that purpose.
The causal role of RVs other than influenza has not been determined in adult patients with pneumonia, and effective treatment with antiviral agents is largely unavailable in clinical practice. Thus, the detection of RVs other than influenza may be regarded as unnecessary for adult patients with pneumonia, especially during the non-flu season considering the fact that the multiplex RT-PCR test represented only 0.8% of sputum bacterial culture tests. However, clinical studies of pneumonia associated with RVs other than influenza have reported increased infection rates in both immunocompromised and non-immunocompromised patients [3,5,6,11,12,13,14]. Additionally, a few antiviral agents are recommended for severe pneumonia caused by RVs other than influenza, especially in immunocompromised patients [3,15]. In this study, although most of the tests were performed for the diagnosis of influenza infection during the flu season, RVs other than influenza were found in 45.4% (20/44) of the patients with RV-associated pneumonia. Furthermore, in more than half (6/9) of the patients with a bacterial/viral coinfection, RVs other than influenza, including RSV, HMPV, and HRV, were detected. This group of coinfected patients had higher rates of mortality than either the group with a single RV infection or the group without an RV infection. Although the association with higher mortality may be due to more serious underlying diseases such as hematologic malignancy (22.2%) and the impact of bacterial infections in this group, the significance of RVs other than influenza should not be neglected. The clinical significance of RSV, HMPV, and HRV in adult patients with pneumonia has recently been reported [16,17,18,19]. Our study suggests that RVs other than influenza should be considered in adult patients with pneumonia, and that the clinical impact of these RVs should be evaluated in future clinical studies.
In our study, 20.3% of patients with an NRI had positive multiplex RT-PCR test results. The clinical presentation of an RV infection may vary according either to the pathogenic potential of the RV or the degree of host immunity against RVs, and respiratory symptoms or signs in some patients with an RV infection may be minimal or absent. Asymptomatic carriers of RVs are another possible explanation, as suggested in a previous study [11].
This study has several limitations. First, it was not designed to compare the clinical management, outcomes, and medical costs of patients who underwent multiplex RT-PCR testing with patients who did not. Thus, our study does not include direct evidence for the clinical usefulness of the multiplex RT-PCR test for RVs. Prior studies have shown conflicting results for the cost-effectiveness of the multiplex RT-PCR test [4]. Additional studies are required before the multiplex RT-PCR test for RVs can be strongly recommended in South Korea. Second, the platform for multiplex RT-PCR testing was changed during the study period, which might have affected the multiplex RT-PCR results and the positivity rates. However, the RV7 test was performed only in 24.0% of 291 cases. Third, clinical data were retrospectively collected. Unrecognized clinical factors may have resulted in biases in the study analysis. Fourth, our data cannot be generalized to other centers with different characteristics.
In conclusion, the multiplex RT-PCR test was performed most frequently for adult patients admitted for community-acquired respiratory infections during the flu season at a tertiary care center. The test was performed infrequently, and reporting of the test results was often delayed. The utilization of multiplex RT-PCR testing should be encouraged to more effectively diagnose infections with influenza and other RVs, both inside and outside the hospital.
KEY MESSAGE
In adult patients, multiplex reverse transcription-polymerase chain reaction test for respiratory viruses was infrequently performed and the utilization of the test was limited for the diagnosis of influenza infection.
Considering the growing importance of other respiratory viruses except influenza, the utilizaiton of the test may be encouraged for more adult patients with acute respiratory illness.
Notes
No potential conflict of interest relevant to this article was reported.