High serum and urine neutrophil gelatinaseassociated lipocalin levels are independent predictors of renal progression in patients with immunoglobulin A nephropathy
Article information
Abstract
Background/Aims
Tubulointerstitial injury plays an important role in the progression of immunoglobulin A nephropathy (IgAN), and neutrophil gelatinase-associated lipocalin (NGAL) is among the most sensitive tubular biomarkers. We investigated whether serum or urine NGAL predicts prognosis in patients with IgAN.
Methods
The present study enrolled patients with biopsy-proven IgAN from January 2005 to December 2010, whose serum and urine samples at the time of kidney biopsy were preserved by freezing. We retrospectively reviewed patient clinical data and followed patients until October 2012. Serum and urine NGAL levels were measured using an enzyme-linked immunosorbent assay kit. Renal progression was defined as an estimated glomerular filtration rate decline by > 50% or progression to end-stage renal disease.
Results
There were 121 patients enrolled in this study. During the median follow-up period of 41.49 months, renal progression was found in nine patients (7.4%). Serum or urine NGAL alone could not predict renal progression; however, when serum and urine NGAL levels were combined, belonging to the high NGAL group independently predicted renal progression (hazard ratio [HR], 5.56; 95% confidence interval [CI], 1.42 to 21.73; p = 0.014), along with tubular damage graded according to the Oxford classification as T2 (HR, 8.79; 95% CI, 2.01 to 38.51; p = 0.004). In addition, a Kaplan-Meier curve of renal survival showed significantly higher renal progression in patients in the high NGAL group (log rank, p = 0.004).
Conclusions
In patients with IgAN, high serum and urine NGAL levels at the time of kidney biopsy predict renal progression.
INTRODUCTION
Immunoglobulin A nephropathy (IgAN) is the most prevalent glomerular disease in most countries, and shows a diverse clinical course. Previous large-scale studies reported that 25% of patients will have end-stage renal disease (ESRD) 20 years after diagnosis, and an additional 20% will have progressive impairment of renal function [1]. According to the Kidney Disease: Improving Global Outcomes (KDIGO) guideline of 2012, proteinuria, blood pressure, and kidney biopsy findings at presentation are associated with risk of ESRD and the doubling of serum creatinine levels [2]. Among these, proteinuria > 1 g/day is a powerful independent predictor of accelerated renal function decline [3,4]. Recently, Working Group of the International IgA Nephropathy Network and the Renal Pathology Society [5] reported that tubulointerstitial injury at kidney biopsy played an important role in the progression of IgAN, and several studies have focused on tubular biomarkers for the prediction of renal progression in patients with IgAN [6,7,8].
Neutrophil gelatinase-associated lipocalin (NGAL) is a 25 kDa acute-phase protein originally purified from human neutrophils that were markedly induced in injured epithelial cells [9]. When tubule damage occurs, NGAL mRNA expression is significantly upregulated in the distal nephron segment for cellular proliferation and differentiation [10,11]. Increased NGAL protein is released into the circulation, freely filtered by the glomerulus and reabsorbed in the proximal tubules [6]. Thus, following tubular damage, both serum and urine NGAL excretion is increased.
Urinary NGAL is closely associated with the biopsy findings of early tubulointerstitial injury in IgAN [8]. However, no study determined the prognostic significance of serum or urine NGAL in patients with IgAN. In this study, we investigated whether serum or urine NGAL predicts prognosis in patients with IgAN.
METHODS
Subjects
We performed a retrospective analysis of adult patients who were diagnosed with IgAN by kidney biopsy between January 2005 and December 2010 at Pusan National University Hospital, Busan, Korea. We excluded patients with other causes of IgA-positive glomerular staining (Henoch-Schonlein purpura or liver disease), diabetes, acute and chronic persistent infection, thyroid dysfunction, neoplasm, or chronic inflammatory disorders. All of the serum and urine samples at the time of kidney biopsy were preserved in a frozen state according to the standard kidney biopsy protocol of our clinic. Local ethics committee approval was obtained for the anonymous analyses of routinely collected clinical data, with a waiver of informed consent (E-2012119).
Data collection and patient follow-up
Patients regularly visited the clinic at intervals of 2 to 4 months after their diagnosis of IgAN. Baseline data and data during the follow-up periods were collected. The parameters collected at baseline included gender, age, history of hypertension, and biochemical data at the time of kidney biopsy and total protein, albumin, serum blood urea nitrogen (BUN), serum creatinine, and the spot urine protein/creatinine ratio (PCR). Blood and urine samples on the day of kidney biopsy were preserved, and the samples were centrifuged at 3,000 rpm for 10 minutes, and stored at -80℃ until assayed. Follow-up laboratory data included serum BUN and creatinine. The estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [12], and renal progression was defined as an eGFR decline > 50% from baseline or progression to ESRD. For patients with renal progression, the follow-up period was calculated as the interval between kidney biopsy and the first time point of recognized renal progression. For patients without renal progression, the period was calculated as the interval between kidney biopsy and the last visit. The kidney biopsy specimens were re-evaluated by a pathologist blinded to the patients' outcome. The histologic findings were classified according to the Oxford classification criteria [5]. In this classification, tubule and interstitial status were classified according to the extent of tubule atrophy/interstitial fibrosis: T0 < 25%; T1 25% to 50%; T2 > 50%.
Measurements of serum and urine NGAL
Both serum and urine NGAL were measured using an enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA) based on the manufacturer's instructions. The interassay and intra-assay variability for serum and urine NGAL were 5.6% to 7.9% and 3.6% to 4.4%, respectively. The detection limit of the assay was 0.012 ng/mL. The spot urine level of NGAL was normalized to the urinary creatinine concentration (NGAL/Cr).
Definition of serum and urine NGAL levels
High serum NGAL was defined as a level > 150 ng/mL, in accordance with the reference value of serum NGAL at our clinic. As the reference value of the urine NGAL/Cr ratio had not been established in our clinic, we defined high urine NGAL/Cr as a level greater than the median value of our cohort. We further classified patients whose serum and urine NGAL levels were both elevated as those in the high NGAL group.
Statistical analysis
The data were analyzed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). For continuous variables, the means ± standard deviation were used for normally distributed data; otherwise, the median was used. Differences between the two groups were assessed using Student t test or the Mann-Whitney U test for continuous variables; the chi-square test was used for categorical variables. Differences between baseline and follow-up data were assessed using a paired t test. Differences between the three groups were assessed using the one-way analysis of variance test or Kruskal-Wallis test. Pearson or Spearman correlation coefficients were used as appropriate to test for correlations between tubulointerstitial damage and other variables. To evaluate prognostic factors for renal progression, non-parametric variables were Ln-transformed to achieve normality; after transformation of the variables, we performed univariable and multivariable analyses using Cox regression analysis. A multivariable analysis was performed with two different models. In model I, age, gender, the presence of hypertension, use of steroids, use of immunosuppressants, serum albumin, eGFR, serum Ln-NGAL, urine PCR, urine Ln-NGAL/Cr, and T2 were adjusted. In model II, the high NGAL group was adjusted instead of serum Ln-NGAL and urine Ln-NGAL/Cr. To illustrate the effect of the high NGAL group on renal progression, a Kaplan-Meier curve for cumulative renal survival was constructed. A p < 0.05 was considered statistically significant.
RESULTS
Patient characteristics and histopathologic features
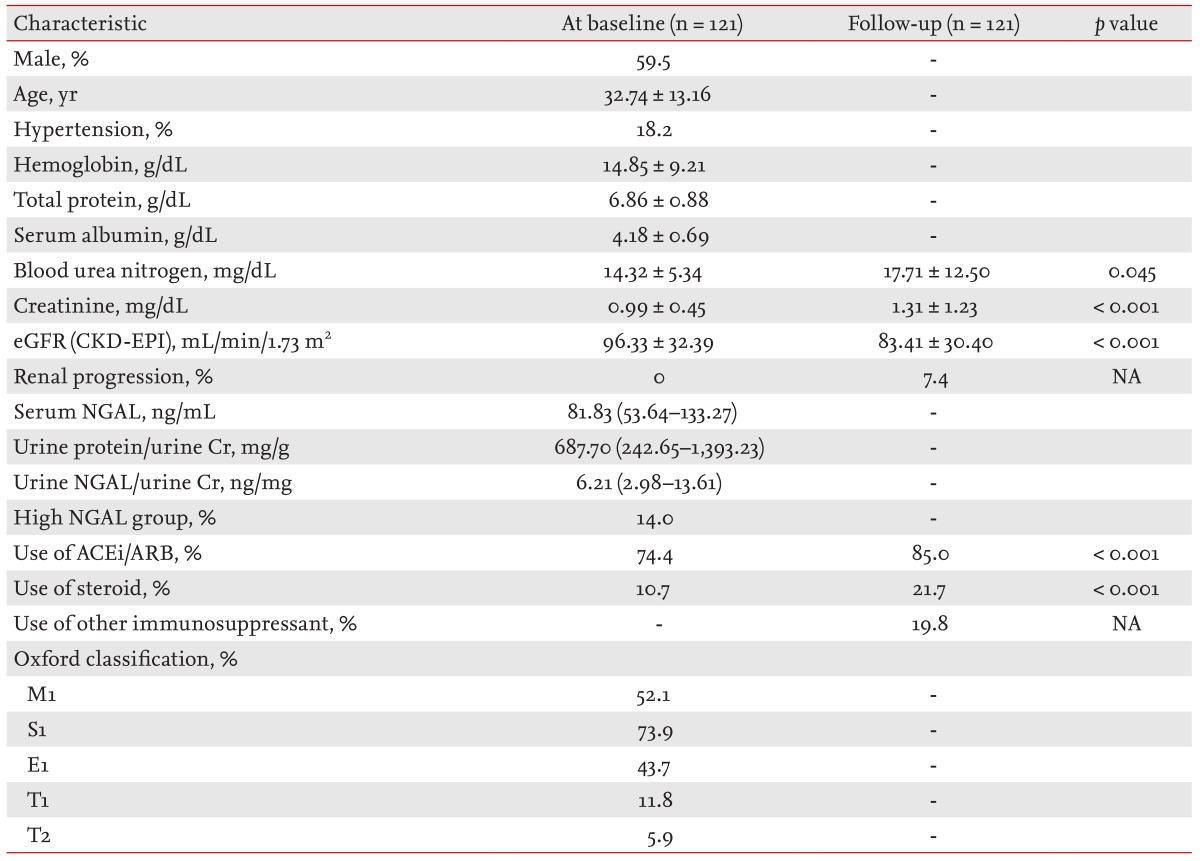
A total of 121 patients were enrolled in this study (Fig. 1). The patients' mean age was 32.74 ± 13.16 years, and 59.5% of the patients were male. Of the patients, 18.2% had hypertension at baseline, and the mean serum creatinine levels and eGFR were 0.99 ± 0.45 mg/dL and 96.33 ± 32.39 mL/min/1.73 m2, respectively. The median serum NGAL level was 81.83 ng/mL (range, 53.64 to 133.27). All of the patients had microscopic hematuria, and their median urine PCR was 687.70 mg/g (range, 242.65 to 1,393.23). The median level of urinary NGAL/Cr was 6.21 ng/mg (range, 2.98 to 13.61). In our cohort, 14.0% of the patients were classified as belonging to the high NGAL group. The other baseline characteristics and distribution of histologic lesions according to the Oxford classification are summarized in Table 1.
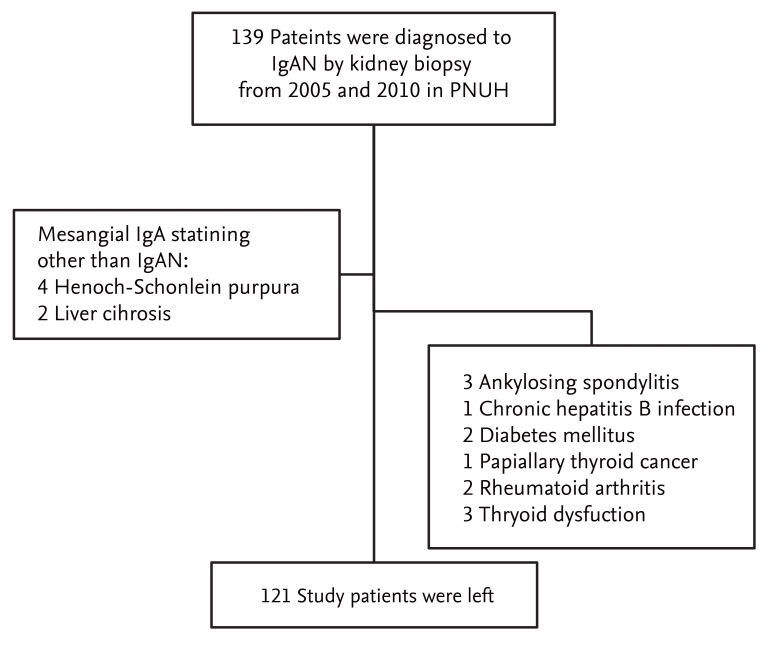
Flow chart diagramming the patient enrollment and exclusion processes. IgAN, immunoglobulin A nephropathy; PNUH, Pusan National University Hospital.
Correlations between serum or urine NGAL and baseline clinical/histological parameters
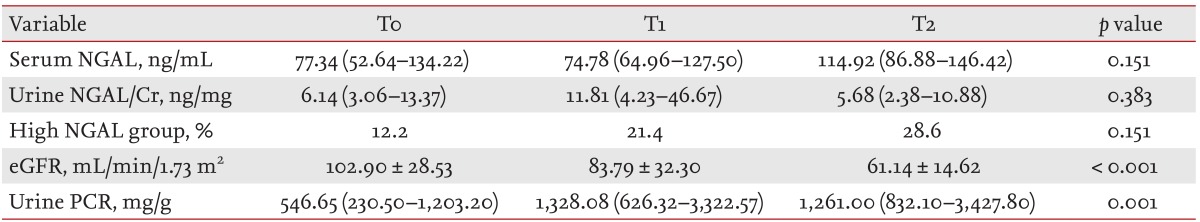
Serum NGAL was negatively correlated with baseline eGFR (r = -0.225, p = 0.015), and was positively correlated with baseline urine PCR (r = 0.201, p = 0.030). Urine NGAL/Cr was negatively correlated with baseline eGFR (r = -0.186, p = 0.045) and baseline serum albumin (r = -0.315, p = 0.001). There was no relationship between serum NGAL and urine NGAL/Cr. Belonging to the high NGAL group was positively correlated with urine PCR (r = 0.280, p = 0.002) and negatively correlated with serum albumin (r = -0.243, p = 0.007), but not with baseline eGFR. When we compared serum and/or urine NGAL levels according to the histologic status of the tubules and interstitium, we could not find any significant increasing or decreasing trend in serum and/or NGAL levels. Only eGFR and urine PCR showed a decreasing and increasing trend in accordance with progressive tubule damage (Table 2).
Treatment of IgAN during the follow-up period
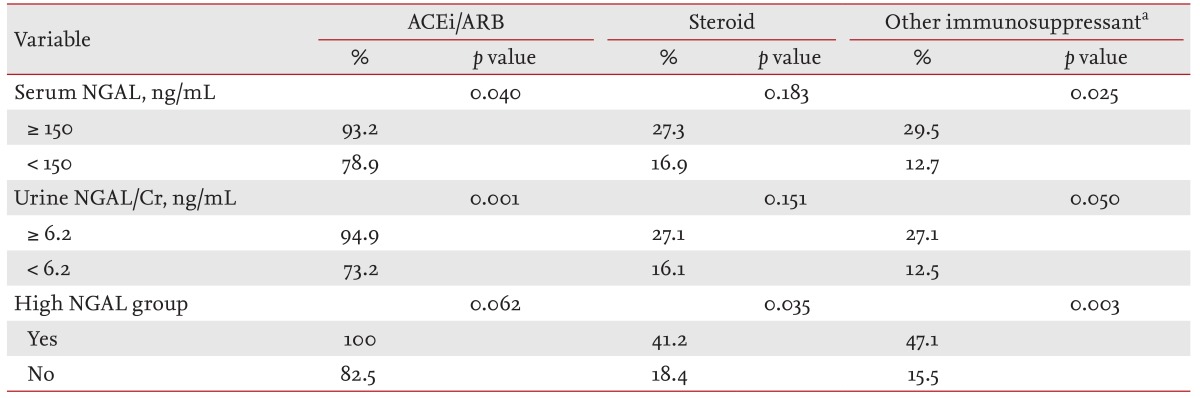
During the median follow-up period of 41.49 months, 85.0% of the patients were prescribed angiotensin-converting-enzyme inhibitor/angiotensin receptor blocker, and steroid treatment was performed in 21.7% of the patients. Immunosuppressants other than steroids were used in 19.8% of the patients. In our cohort, azathioprine, mycophenolate mofetil, and cyclosporine were used. Differences in the use of immunosuppressants according to the serum and/or urine NGAL status are summarized in Table 3. More steroids and immunosuppressants were prescribed to patients in the high NGAL group. Despite these treatments, mean eGFR declined and renal progression was found in nine patients (7.4%) (Table 1).
Predictors of renal progression in patients with IgAN
In our cohort, renal progression was found in nine patients (7.4% of patients). In univariate analysis, lower serum albumin (hazard ratio [HR], 0.32; 95% confidence interval [CI], 0.12 to 0.86; p = 0.024), the high NGAL group (HR, 1.72; 95% CI, 1.25 to 17.79; p = 0.022) and T2 lesions (HR, 9.71; 95% CI, 2.30 to 40.96; p = 0.002) were associated with renal progression. In the multivariate analysis that adjusted for age, gender, the presence of hypertension, use of steroids, use of immunosuppressants, serum albumin, eGFR, serum Ln-NGAL, urine PCR, urine Ln-NGAL/Cr and T2, lower serum albumin and T2 lesions independently predicted renal progression, although serum or urine NGAL levels alone could not predict renal progression. When we adjusted for the high NGAL group instead of serum Ln-NGAL and urine Ln-NGAL/Cr, the high NGAL group and T2 lesions were found to be independent prognostic factors for the prediction of renal progression (Table 4). When the renal survival curve was adjusted, the high NGAL group showed significantly lower renal survival compared to the other patients (Fig. 2).
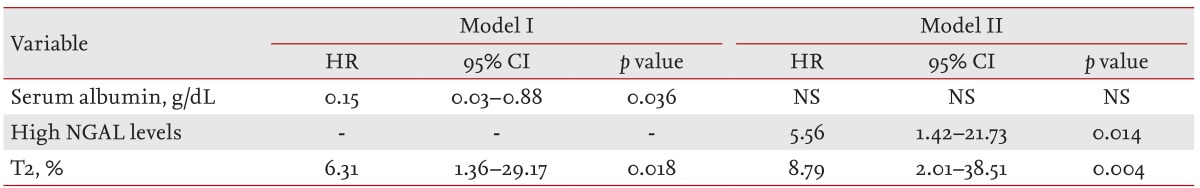
Hazard ratios and confidence intervals of baseline predictors of progression after multivariate regression analysis
DISCUSSION
In patients with IgAN, tubulointerstitial injury is an important predictive factor for renal progression, and NGAL is one of the most representative tubular biomarkers. In our study, advanced tubulointerstitial injury (T2) at kidney biopsy and belonging to the high NGAL group were important prognostic factors for the prediction of renal progression. By our definition, patients in the high NGAL group comprised 14.0% of the cohort. When we compared renal survival between patients in the high NGAL group and other patients, renal progression was significantly increased in the former group. However, the histologic status of the high NGAL group was not associated with advanced tubule injury at biopsy, and only eGFR and urine PCR levels represented tubule injury. Thus, in our cohort, belonging to the high NGAL group predicted renal progression, independent of tubulointerstitial fibrosis.
NGAL was first identified as an early biomarker of acute kidney injury. NGAL is the most upregulated gene in the kidney very early after acute injury in animal models [13,14], and many studies have been published on the predictive value of urine or serum NGAL after cardiac surgery, contrast injection, or kidney transplantation [15,16,17,18,19,20]. Recently, one study reported that serum or urine NGAL had prognostic power for renal progression, even in CKD patients [21]. However, the prognostic significance of serum or urine NGAL has not been clearly identified in the context of IgAN. In the study by Peters et al. [7], various urinary tubule markers, including kidney injury molecule 1 (KIM-1) and NGAL, were studied with regard to their prognostic significance. In that study, urinary KIM-1 was found to be an independent predictor of ESRD in patients with IgAN, whereas urinary NGAL was not a predictor of renal progression [7]. In the present study, urine or serum NGAL was not predictive of renal progression, although advanced tubule injury (T2) significantly predicted renal progression. The commonality of these studies is that urine NGAL levels did not represent advanced tubulointerstitial fibrosis. Although urinary NGAL was positively correlated with early tubulointerstitial injury (Lee grade II, III) in the study by Ding et al. [8], the authors did not report its relationship to advanced tubulointerstitial injury (Lee grade IV, V). Peters et al. [7] analyzed the association of all staged tubulointerstitial damage (Oxford T0 to T2) with NGAL excretion, and the urinary NGAL level did not correlate with tubulointerstitial score. In the present study, the urine NGAL/Cr level peaked at T1; for T2, its level decreased to that of T0. This finding suggested that urinary NGAL excretion only reflects early tubule damage and cannot reflect advanced tubule damage, as NGAL mRNA is no longer expressed in response to tubule damage by atrophic tubular cells [10,11,22].
We found that serum NGAL level was positively correlated with eGFR. As reported previously, serum NGAL is elevated in patients with renal dysfunction [23,24,25], and renal dysfunction at presentation has consistently been related to the risk of ESRD. However, several previous studies have reported that an initial lower GFR was not accompanied by a faster rate of kidney function decline [26,27]. Thus, in KIDGO 2012, experts commented that, "proteinuria, blood pressure, and pathological features should take precedence over initial GFR in the estimation of the future rate of kidney function decline" [2]. For this reason, it is important to distinguish those patients whose disease is likely to continuously progress to ESRD, even patients with renal dysfunction at presentation. In our study, serum NGAL alone could not predict renal progression, whereas high levels of NGAL did successfully predict renal progression. Although the exact mechanism of this finding is not clear, it is possible that the combination of serum and urine NGAL levels provided additional information on the tubular process involved in progressive renal failure. Thus, increased serum NGAL represents decreased renal function [23,24,25], and increased urine NGAL/Cr represents ongoing tubule damage [10,11,22].
Our study had several limitations. First, the total follow-up period was relatively short, and the number of patients with renal progression was relatively small. Thus, our results should be confirmed in a large population with a long-term follow-up period. Second, these data could not precisely consider the treatment modality in the analysis of the predictors of renal progression, because the dose and treatment duration were heterogeneous. However, this study is still important, as it is the first report to reveal the additive role of serum and urine NGAL in the prediction of renal progression in patients with IgAN.
In conclusion, NGAL is a sensitive biomarker of tubule damage, and advanced tubule/interstitial injury is a well-known predictor of renal progression. However, in patients with IgAN, serum or urine NGAL levels do not represent the extent of tubulointerstitial fibrosis. In addition, serum NGAL and urine NGAL/Cr levels could not predict renal progression. However, a combined analysis of serum and urine NGAL levels successfully predicted renal progression in patients with IgAN in our cohort. This finding indicates that the combined elevation of serum and urine NGAL excretion provides additive information on tubular processes in the renal progression in patients with IgAN.
KEY MESSAGE
Advanced tubulointerstitial injury at baseline is associated with renal progression in patients with immunoglobulin A nephropathy (IgAN).
Serum or urine neutrophil gelatinase-associated lipocalin (NGAL) levels are well-known tubular biomarkers; however, in patients with IgAN, the levels of these markers do not represent advanced tubulointerstitial fibrosis. In addition, serum NGAL or urine NGAL/Cr levels could not predict renal progression.
In patients with IgAN, combined elevation of serum and urine NGAL levels successfully predicted renal progression. This finding implies that, a combination of these markers provides additive information on tubular process on the renal progression.
Acknowledgments
This study was supported by 2014 Clinical Research Grant from Pusan National University Hospital. We sincerely thank Dr. Keun Hyeun Lee at Hemin Korean Traditional Medical Clinic for collecting the data.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.