Oral udenafil and aceclofenac for the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis in high-risk patients: a randomized multicenter study
Article information
Abstract
Background/Aims:
Acute pancreatitis is a common complication of endoscopic retrograde cholangiopancreatography (ERCP). Combination therapy w ith ora l udenafil and aceclofenac may reduce the occurrence of post-ERCP pancreatitis by targeting different pathophysiological mechanisms. We investigated whether combining udenafil and aceclofenac reduced the rates of post-ERCP pancreatitis.
Methods:
A prospective, randomized, double-blind, placebo-controlled, multicenter study was conducted in four academic medical centers. Between January 2012 and June 2013, a total of 216 patients who underwent ERCP were analyzed for the occurrence of post-ERCP pancreatitis. Patients were determined to be at high risk for pancreatitis based on validated patient and procedure-related risk factors.
Results:
Demographic features, indications for ERCP, and therapeutic procedures were similar in each group. There were no significant differences in the rate (15.8% [17/107] vs. 16.5% [18/109], p = 0.901) and severity of post-ERCP pancreatitis between the udenafil/aceclofenac and placebo groups. One patient in each group developed severe pancreatitis. Multivariate analyses indicated that suspected dysfunction of the sphincter of Oddi and endoscopic papillary balloon dilation without sphincterotomy were associated with post-ERCP pancreatitis.
Conclusions:
Combination therapy with udenafil and aceclofenac is not effective for the prevention of post-ERCP pancreatitis.
INTRODUCTION
Acute pancreatitis remains the most common adverse event of endoscopic retrograde cholangiopancreatography (ERCP), with an incidence ranging from 2% to 4% in low-risk patients to 8% to 20% in high-risk patients [1]. Post-ERCP pancreatitis is generally mild but can be fatal in some cases [2]. A number of preventive measures have been studied in an effort to reduce the incidence of post-ERCP pancreatitis [1]. Rectal nonsteroidal anti-inflammatory drugs (NSAIDs) and placement of pancreatic stents have been shown to be preventive in some studies [3,4].
Understanding the pathogenesis of post-ERCP pancreatitis is important for its prevention. The pathophysiology of post-ERCP pancreatitis is still uncertain but is believed to be multifactorial [1]. Thus, pharmacologic intervention targeting a single causative factor may not be sufficient to prevent pancreatitis; several different factors may need to be targeted. Phosphodiesterase type 5 (PDE-5) inhibitors are novel agents that reduce the basal pressure of the sphincter of Oddi [2]. The administration of a PDE-5 inhibitor before ERCP may decrease the tone of the sphincter of Oddi, allow for easier cannulation, and ultimately reduce the occurrence of post-ERCP pancreatitis [5]. However, use of a low dose alone does not significantly decrease the rate of post-ERCP pancreatitis [2]. NSAIDs are potent inhibitors of phospholipase A2 that block the inflammatory cascade in the pathogenesis of acute pancreatitis [6,7]. The available data suggest that the administration of NSAIDs can reduce the incidence of post-ERCP pancreatitis, although individual trials have reported conflicting results depending on the NSAID used and the route of administration [8]. The dual actions of high-dose PDE-5 inhibitors and NSAIDs may be expected to result in efficient cannulation and early blockade of inflammation. Thus, we investigated whether oral administration of a combination of a high dose of the PDE-5 inhibitor udenafil and the NSAID aceclofenac would reduce the occurrence of acute pancreatitis in patients at high risk for post-ERCP pancreatitis.
METHODS
This study was performed at four Korean academic centers (Chung-Ang University Hospital, Konkuk University Medical Center, Inje University Busan Paik Hospital, and Inje University Ilsan Paik Hospital) between January 2012 and June 2013. The study protocol was approved by the Institutional Review Boards at all participating centers. All patients provided informed consent before enrollment. The protocol was registered at http://cris.nih.go.kr with the identifier KCT0000203.
Patients
Enrolled patients ranged from 20 to 80 years of age and had appropriate indications for therapeutic or diagnostic ERCP because of suspected pancreatobiliary disease. Patients were eligible if they had one or more of the following high-risk factors: clinical suspicion of sphincter of Oddi dysfunction (SOD), extrahepatic bile duct diameter ≤ 10 mm, or planned manipulation of the pancreatic duct. Patients were excluded from the study for any of the following reasons: need for emergent ERCP; history of a biliary/pancreatic sphincterotomy or balloon sphincteroplasty; a surgically altered biliary anatomy; a diagnosis of acute active pancreatitis 72 hours before ERCP; administration of a nitric oxide donor such as nitroglycerin or isosorbide mononitrate or dinitrate; a recent history of cerebrovascular or coronary events, coronary bypass surgery, cardiac failure, or life-threatening arrhythmia within 6 months; a history of hypersensitivity reactions to PDE-5 inhibitors or NSAIDs; chronic renal failure; and current use of NSAIDs other than cardioprotective aspirin.
Study design
The study was designed as a randomized, placebo controlled, double-blind trial. A total of 2,066 patients who were indicated for ERCP were pre-screened. To this end, we conducted a physical examination, a radiological examination, a comprehensive review of blood tests, a 12-lead electrocardiogram, and a thorough medical history on previous procedures in the pancreatic and biliary ducts, previous acute pancreatitis, drug therapy, and systemic diseases. In total, 216 patients were enrolled and randomized in a 1:1 ratio to receive the PDE-5 inhibitor udenafil (Zydena 200 mg, Dong-A Pharmaceutical Co., Seoul, Korea) and aceclofenac (Acrofen 100 mg, Dong-A Pharmaceutical Co.), or placebo, using an individual center-based, computer-generated, block randomization (size 10), random-number list. Udenafil and aceclofenac tablets or placebo tablets, which were identical in appearance, were placed in a concealed envelope and sorted sequentially according to the randomization schedule.
Enrolled patients took their allocated tablets 2 hours before ERCP, and blood pressure and oxygen saturation were monitored for 4 hours after ERCP. ERCP procedures were performed by six experienced endoscopists who perform more than 150 ERCPs annually. The endoscopists included the following data: specific details concerning the procedure, including the presence of patulous papilla, time to deep common bile duct (CBD) cannulation, number of cannulations into the pancreatic duct, extent of pancreatic opacification, and type of therapy performed.
All patients were prospectively monitored in the hospital for the development of post-ERCP pancreatitis for at least 24 hours after ERCP. Blood was obtained to determine the levels of amylase and lipase in serum at 24 hours, and if necessary, at 48 hours, after ERCP.
Definitions
SOD was defined according to the Hogan-Geenen SOD classification system [9]. A papillary orifice was arbitrarily defined as patulous when bile or pancreatic fluid gushed out spontaneously through the opened orifice [2]. The number of cannulation attempts at the papilla with a cannulation instrument was counted during the procedure. Cannulation difficulty was defined as easy when 1 to 10 attempts were needed, and difficult when > 10 attempts were needed. The diameter of the bile duct was measured at the level of the hilum, adjusted for radiographic magnification [2]. The number of pancreatic duct injections was defined as the total number of times any volume of contrast was injected into the pancreatic duct. The extent of opacification seen on the pancreatography was characterized as partial or complete filling.
The definition of post-ERCP complications and the grading of their severity were based on consensus criteria [10]. Post-ERCP pancreatitis was diagnosed when new-onset or increased abdominal pain caused a hospital admission or prolonged hospital stay and was associated with an increase in amylase levels of at least 3-fold above normal 24 hours after the procedure. Pancreatitis severity was graded as mild for patients with up to 3 additional hospital days and moderate for those staying between 4 and 10 days. Pancreatitis was graded as severe in patients who spent more than 10 days in the hospital or if any of the following occurred: hemorrhagic pancreatitis, pancreatic necrosis, development of a pancreatic pseudocyst, or the need for percutaneous drainage or surgery.
Outcome measures
In this prospective, randomized, controlled study, the primary outcome measure was the effect of the combination of two agents, acting at different levels of the acute pancreatitis cascade, in terms of preventing the development of post-ERCP pancreatitis. The secondary outcome measures were the severity of post-ERCP pancreatitis, the side effects induced by udenafil and aceclofenac, the number of cannulation attempts, and the success rate of intended selective cannulations.
Statistical analysis
Power analysis (α = 0.05 in a two-sided chi-square test; power = 0.80) determined that 108 patients were required in each group to demonstrate a decrease in the rates of post-ERCP pancreatitis from 20% to 7% with udenafil and aceclofenac therapy. We added five patients to each arm of the study to account for the loss of some patients. Patient and procedure characteristics were compared between the two groups using the chi-square and Fisher exact tests for categorical variables and the Student t test for continuous variables. The proportions of patients with post-ERCP pancreatitis were compared to the chi-square or Fisher exact tests. Select patient- or procedure-related characteristics were tested by univariate analysis as potential risk factors for post-ERCP pancreatitis using logistic regression. A multivariate analysis was performed using factors that had univariate p values of < 0.20 on logistic regression with a backward likelihood ratio. A p < 0.05 was considered to be significant. Results from multivariate logistic regression analysis were considered definitive because it determined variables independently associated with post-ERCP pancreatitis after adjusting for the contributions of the other variables. Therefore, univariate statistical tests should be taken as descriptive only because these p values were not corrected for multiple testing of outcome data arising from individual patients. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
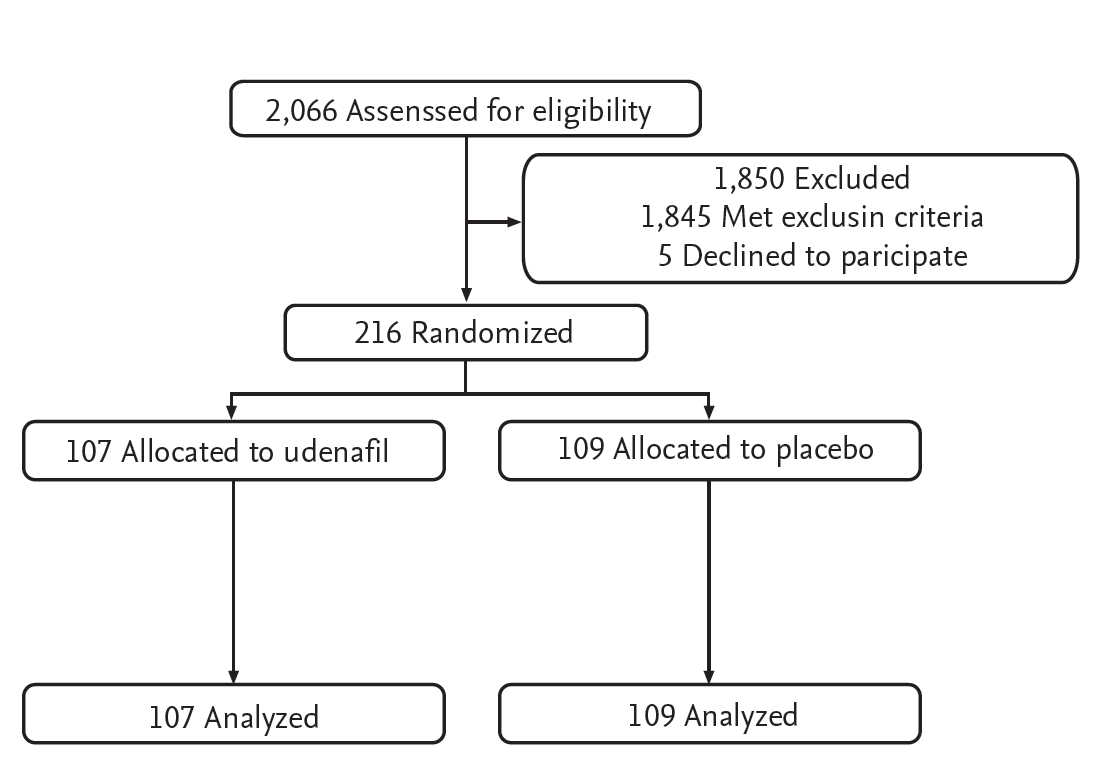
A total of 2,066 patients were pre-screened, and 1,850 patients were excluded before enrollment because of prior sphincterotomy or sphincteroplasty (n = 684), surgically altered biliary anatomy (n = 137), acute active pancreatitis (n = 94), contraindication to PDE-5 inhibitors or NSAIDs (n = 128), recent history of a cerebral or cardiovascular event or surgery (n = 81), need for emergency ERCP (n = 193), refusal to participate (n = 5), or extrahepatic bile duct diameter > 10 mm (n = 528). A total of 216 patients were enrolled in the study and randomized; 107 received udenafil/aceclofenac and 109 received placebo. All 216 patients completed the study, including 107 patients in the udenafil/aceclofenac group and 109 patients in the placebo group (Fig. 1).
Patient and ERCP characteristics
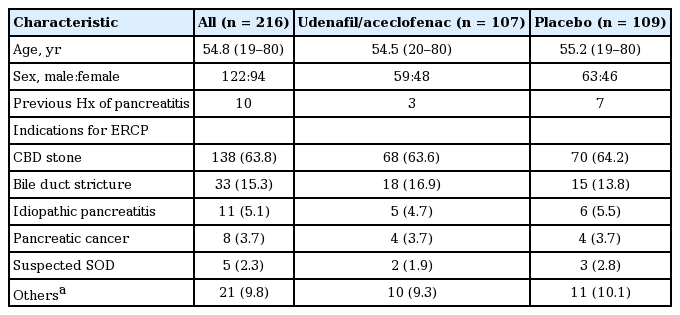
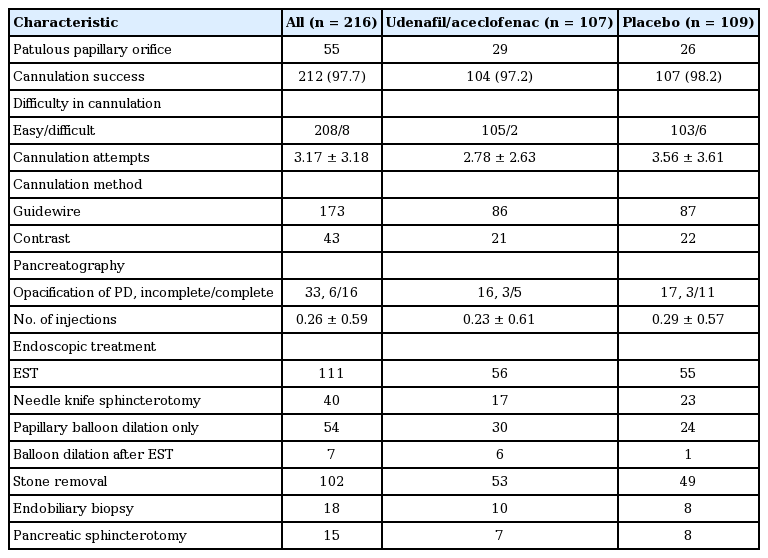
No significant differences in patient characteristics and ERCP indications were found between the two groups (Table 1). There were 48 females in the udenafil/aceclofenac group and 46 females in the placebo group, and their mean ages were 54.5 and 55.2 years, respectively. In addition, both groups were comparable regarding ERCP findings and endoscopic procedures (Table 2). A CBD stone was the most common indication for ERCP (n = 138, 63.8%). All patients had at least one of the following predefined patient- or procedure-related risk factors: clinical suspicion of SOD (n = 5), extrahepatic bile duct diameter ≤ 10mm (n = 196), and planned manipulation of the pancreatic duct (n = 15). The overall success rate of intended selective cannulation was 97.7%, and there were no significant differences in cannulation success rates between the two groups. The mean number of cannulation attempts was lower in the udenafil/aceclofenac group than in the placebo group (2.78 [range, 1 to 16] vs. 3.56 [range, 1 to 16], p = 0.132). Unintended cannulation into the pancreatic duct was more frequently observed in the placebo group than in the udenafil/aceclofenac group (42.2% vs. 30.8%, p = 0.08).
Post-ERCP pancreatitis
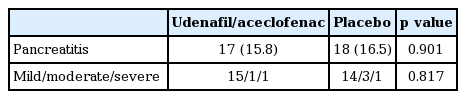
Post-ERCP pancreatitis occurred in 35 patients (16.2%) including 17 in the udenafil/aceclofenac group and 18 in the placebo group (15.8% vs. 16.5%, respectively) (Table 3). There were no significant differences in the rate of post-ERCP pancreatitis between the two groups. The severity of post-ERCP pancreatitis was graded as mild in 29 patients, moderate in four patients, and severe in two patients. There were no significant differences in post-ERCP pancreatitis severity between the two groups, and severe pancreatitis developed in one patient in each group. All patients with post-ERCP pancreatitis were treated conservatively and recovered without sequelae.
Adverse effects
Adverse effects were mild and self-limited in 20 (9.3%) of 216 patients, including 13 in the udenafil/aceclofenac group and seven in the placebo group. In the udenafil/aceclofenac group, facial flushing and headache occurred in 12 patients and hypotension occurred in one patient. In the placebo group, headache and sweating occurred in seven patients. No aceclofenac-related adverse events occurred.
Risk factors for post-ERCP pancreatitis
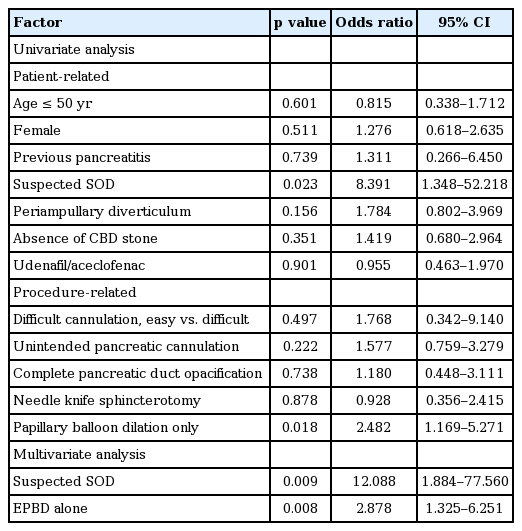
A univariate analysis of risk factors for post-ERCP pancreatitis identified suspected SOD as a patient-related risk factor and endoscopic papillary balloon dilation without sphincterotomy as a procedure-related risk factor. In the multivariate analysis, suspected SOD and endoscopic papillary balloon dilation without sphincterotomy were associated with post-ERCP pancreatitis (Table 4).
DISCUSSION
Pharmacological prevention of post-ERCP pancreatitis has focused on several postulated mechanisms of injury including relaxation of the sphincter of Oddi by calcium channel antagonists [11] and PDE-5 inhibitors [12], interruption of the inflammatory cascade by NSAIDs [13], and inhibition of pancreatic secretion by somatostatin [14] and octreotide [15]. Theoretically, intervention by different agents targeting multiple steps in the pathogenesis of post-ERCP pancreatitis may be more efficient than intervention by a single agent. Our rationale for using udenafil in combination with aceclofenac was based on the ability of this combination to reduce the pressure of the sphincter of Oddi, as well as inflammation present in acute pancreatitis through modulation of the cytokine cascade. However, the combined action of a PDE-5 inhibitor and aceclofenac did not significantly reduce the rate of post-ERCP pancreatitis among the high-risk patients in the current study. The frequency of post-ERCP pancreatitis in the placebo group was 16.5%, higher than that (8.0%) observed in a previous study with unselected patients. The inclusion criteria used in the current study were defined to identify patients who were at risk for post-ERCP pancreatitis. We were successful in that regard, as evidenced by the 16.5% incidence of post-ERCP pancreatitis in the placebo group.
In a previous study that compared udenafil to placebo, there was no significant reduction in the rate of post-ERCP pancreatitis using a low dose (100 mg) of udenafil [2]. As the efficacy of PDE-5 inhibitors such as udenafil is dose-dependent and 100 mg udenafil might be suboptimal to induce sufficient relaxation of the sphincter of Oddi, we used a higher dose in this study (200 mg). However, there were no significant differences in either the number of cannulation attempts or the rate of post-ERCP pancreatitis. The mean number of cannulation attempts before successful cannulation was only 3.4. This may be attributed to the expertise of the endoscopists and lack of trainee involvement in the procedures. On the other hand, although patients with bile duct dilation and stones were excluded from the study, a high prevalence of patients with choledocholithiasis may make conditions less suited for a PDE-5 inhibitor to exert its relaxation effect on the functionally altered sphincter of Oddi owing to fibrosis [2].
Rectal NSAIDs are effective for preventing post-ERCP pancreatitis [3,16], whereas oral NSAIDs are not [17]. The possible explanation for this difference is that peak NSAID concentrations in plasma are reached in 30 minutes with rectal administration compared to 2 hours with oral administration [16,18]. Although we administered NSAIDs 2 hours before ERCP to ensure peak concentrations, oral administration did not effectively inhibit the initial pathophysiological process that eventually results in post-ERCP pancreatitis [16].
Suspected SOD and endoscopic papillary balloon dilation without sphincterotomy were significantly associated with post-ERCP pancreatitis. Edematous change after balloon dilation without sphincterotomy may cause obstruction of the pancreatic duct orifice that may not be relieved by the PDE-5 inhibitor-induced relaxation of the sphincter of Oddi.
This study had a number of limitations. First, oral NSAIDs were used instead of rectal suppositories, which have been reported to be effective for the prevention of pancreatitis. Unfortunately, rectal NSAIDs are not commercially available in Korea. Second, the actual difference in the rate of post-ERCP pancreatitis was much lower than the expected difference. A prohibitively large sample size (more than 250 patients in each group) would be required to detect a 50% reduction in the occurrence of post-ERCP pancreatitis with udenafil/aceclofenac treatment, assuming the occurrence in the placebo group is 16%.
In conclusion, treatment with a combination of udenafil and aceclofenac was not effective for the prevention of post-ERCP pancreatitis. Suspected SOD and endoscopic papillary balloon dilation without sphincterotomy were independently associated with post-ERCP pancreatitis.
KEY MESSAGE
1. Combination therapy with oral udenaf il and aceclofenac was not effective for the prevention of post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis in high-risk patients.
2. Supsected sphincter of Oddi dysfunction and endoscopic papillary balloon dilation without sphincterotomy were associated with post-ERCP pancreatitis.
Notes
The authors would like to acknowledge Dong-A Pharmaceutical Co. for supplying udenafil, aceclofenac, and placebo tablets.
Acknowledgements
This work was supported by the Inje Research and Scholarship Foundation to JSC.