A novel PRKAR1A mutation resulting in a splicing variant in a case of Carney complex
Article information
To the Editor,
Carney complex (CNC) is an autosomal-dominant inherited disorder featuring cardiac or cutaneous myxoma, multiple endocrine tumors with endocrine overactivity, psammomatous melanotic schwannomas (PMSs), and spotty pigmentations in the mucosa and skin. Endocrine manifestations of CNC include pituitary adenoma with acromegaly, thyroid tumors, testicular large cell calcifying Sertoli cell tumors (LCCSCTs), ovarian lesions, and primary pigmented nodular adrenal disease (PPNAD). Cushing’s syndrome due to PPNAD is adrenocorticotropic hormone (ACTH)-independent and the most frequent endocrine disorder of CNC (25%) [1]. Since the association of CNC and PRKAR1A mutation was first described, 117 mutations of the PRKAR1A gene have been reported [2]. Here, we describe a Korean case of CNC with PPNAD, and report a novel mutation of the PRKAR1A gene.
A 22-year-old male presented with uncontrolled hypertension and purple striae on his abdomen. Ten years previously, he had undergone brain surgery at another hospital for a 4-cm brain tumor in the right cerebello-pontine angle, which was initially diagnosed as a melanocytoma. There was no evidence of brain tumor recurrence. At that time, a testicular biopsy was also performed due to a feeling of testicular lumps on palpation, and it showed severe atrophy. Skin nodule pathology in left infra-auricular area was reported as myxoid liposarcoma. His father, and elder brother appeared healthy, but his mother had passed away suddenly at age 32 and had also demonstrated skin nodules (Fig. 1A). The patient was 180 cm tall, weighing 76 kg, and his blood pressure was 140/90 mmHg on four antihypertensive medications. He showed typical features of Cushing’s syndrome, such as moon face (Fig. 1B), truncal obesity, purple striae on the abdomen (Fig. 1C), and buffalo hump. Multiple skin nodules were noted on his scalp, left ear lobe, and both arms (Fig. 1D) and there were a few brownish pigmentations on his legs (Fig. 1E). His 24-hour urine free cortisol (UFC) was 287 mg/day (normal range, 20 to 90), while the serum ACTH and cortisol at 8:00 AM were 2.6 pg/mL and 29.37 mg/dL, respectively.
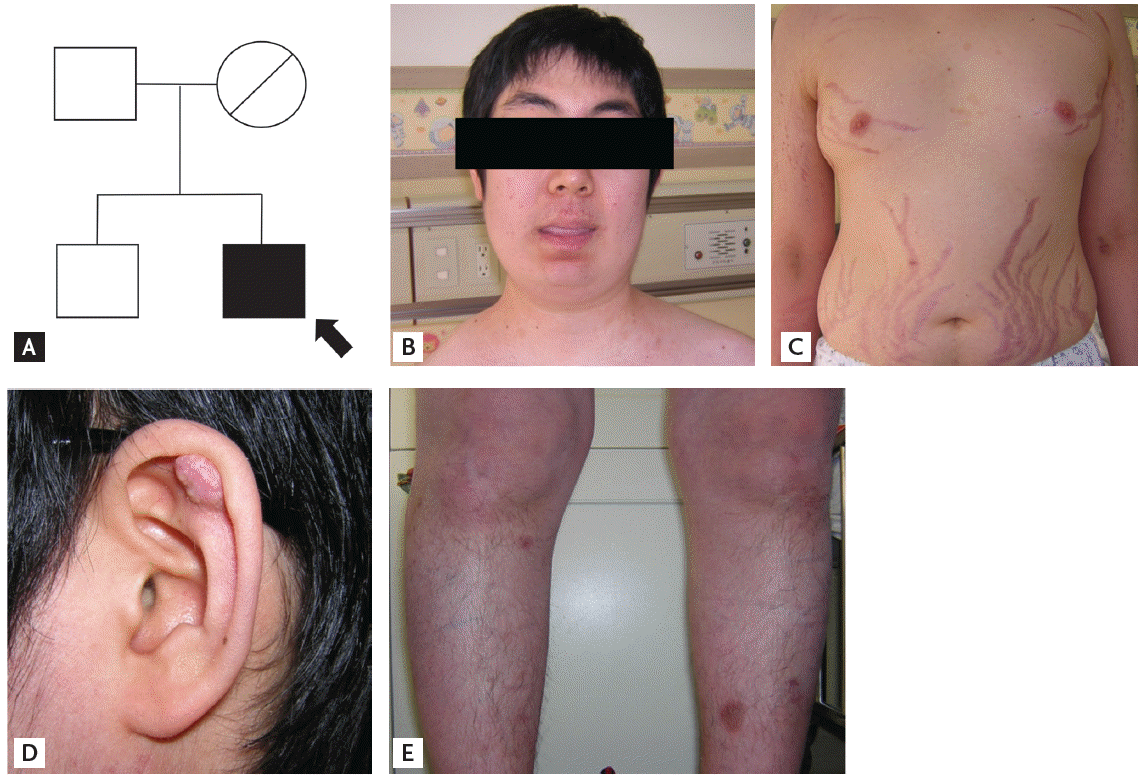
(A) Pedigree of the family with Carney complex. Arrow indicates the proband. Appearance of the patient with Carney complex. (B) Moon face, (C) abdominal obesity with red striae, (D) a skin nodule in the earlobe, and (E) spotty pigmentations on the legs are noted.
During a low-dose dexamethasone suppression test, serum cortisol and 24-hour UFC were not sufficiently suppressed (serum cortisol 33.72 mg/dL, 24-hour UFC 579 mg/day). After a high-dose dexamethasone suppression test, the serum cortisol was elevated to 35.54 mg/dL. The serum cortisol was paradoxically increased after both tests.
A computed tomography scan of the abdomen showed multiple 0.6 to 0.8 cm small nodules in both adrenal glands and a 1.8 cm-sized dominant one on the left (Fig. 2A and 2B). Follicle-stimulating hormone and luteinizing hormone were 2.4 and 7.3 IU/L, respectively, and total testosterone was 0.74 ng/mL. Thyroid function was normal on biochemical testing. Fasting glucose was 104 mg/dL, total cholesterol was 256 mg/dL, and triglycerides were 187 mg/dL, sodium was 145 mEq/L, and potassium was 3.7 mEq/L. Other routine laboratory data were normal. Echocardiogram and thyroid ultrasound showed normal findings.
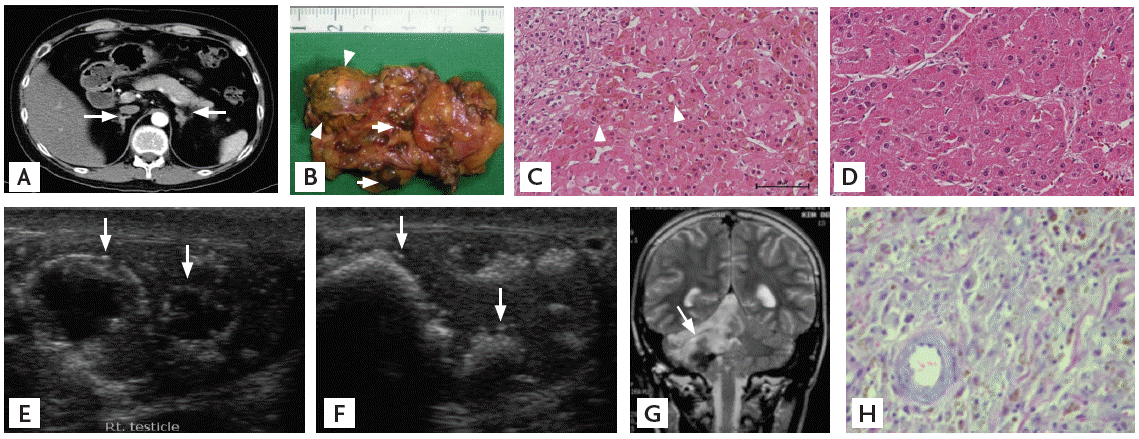
(A) Computed tomography of the abdomen showed multiple small nodules (arrows) with bead-string appearance in both adrenal glands. (B) The gross specimen of the removed left adrenal gland showed multiple small nodules with dark pigmentation (arrows) and a medium-sized nodule without pigmentation (arrowheads), pathologically diagnosed as primary pigmented nodular adrenal disease and an adrenal adenoma, respectively. (C) Pigmentation (arrowheads) of right adrenal micronodule (H&E, ×200). (D) Non-pigmented left adrenal adenoma (H&E, ×200). (E, F) The ultrasonography of right (E) and left (F) testicles showed multiple calcified nodules (arrows), suggesting large cell calcifying Sertoli cell tumors. (G) Previous magnetic reson ance imaging showed a 4-cm mass (arrow) in the right cerebello-pontine angle. (H) Pathology of the brain tumor showed spindle cells containing pigmentation, rediagnosed as a psammomatous melanotic schwannoma (H&E, ×400).
The patient underwent a laparoscopic bilateral adrenalectomy. The histological diagnosis was PPNAD in both adrenal glands, combined with left adrenal adenoma without pigmentation (Fig. 2C and 2D). Following the adrenalectomy, he was prescribed a replacement dose of hydrocortisone and fludrocortisone and his Cushingoid features regressed. A skin nodule in the earlobe was later removed and diagnosed as a myxoma. Ultrasound of the testicles showed multiple calcified nodules replacing the testes, suggesting testicular LCCSCTs (Fig. 2E and 2F). However, he refused surgical removal of the tumors.
We retrospectively reviewed the previous tissue slide of his brain tumor and corrected the diagnosis to PMS (Fig. 2G and 2H). We concluded that he had CNC manifesting as skin myxomas, skin pigmentations, PMS at cerebello-pontine angle, Cushing’s syndrome due to PPNAD, adrenal adenoma, and testicular LCCSCTs.
DNA was extracted from his leukocytes and adrenal tissue obtained from the laparoscopic bilateral adrenalectomy. On DNA sequence analysis of the PRKAR1A gene, we found a novel substitution mutation (c.441-2A>G) in intron 4 (Fig. 3A) from his leukocytes and adrenal tumor tissue without a second hit in PRKAR1A in his adrenal tumor. A mutation in intron 4 could theoretically result in two aberrantly spliced PRKAR1A mRNAs of 41-bp deleted and 62-bp deleted variants (Fig. 3B). To determine whether the products resulting from aberrantly spliced PRKAR1A mRNA were rapidly degraded, real-time polymerase chain reaction was performed to quantify expression of the mRNAs in the leukocytes and the adrenal tumor of the CNC patient. Setting the quantification ratio of the wild-type PRKAR1A mRNA in a control sporadic adrenal tumor at 1.0, the relative quantification ratios of the wild-type PRKAR1A mRNA, 41-bp deleted PRKAR1A mRNA, and 62-bp deleted PRKAR1A mRNA in the CNC were 0.46, 0.00, and 0.08, respectively (Fig. 3C). Studies have not yet been completed for the wild-type and 62-bp deleted PRKAR1A protein expression in this case, but the data may suggest haploinsufficiency of wild-type PRKAR1A mRNA and premature decay of most of the mutant mRNA.
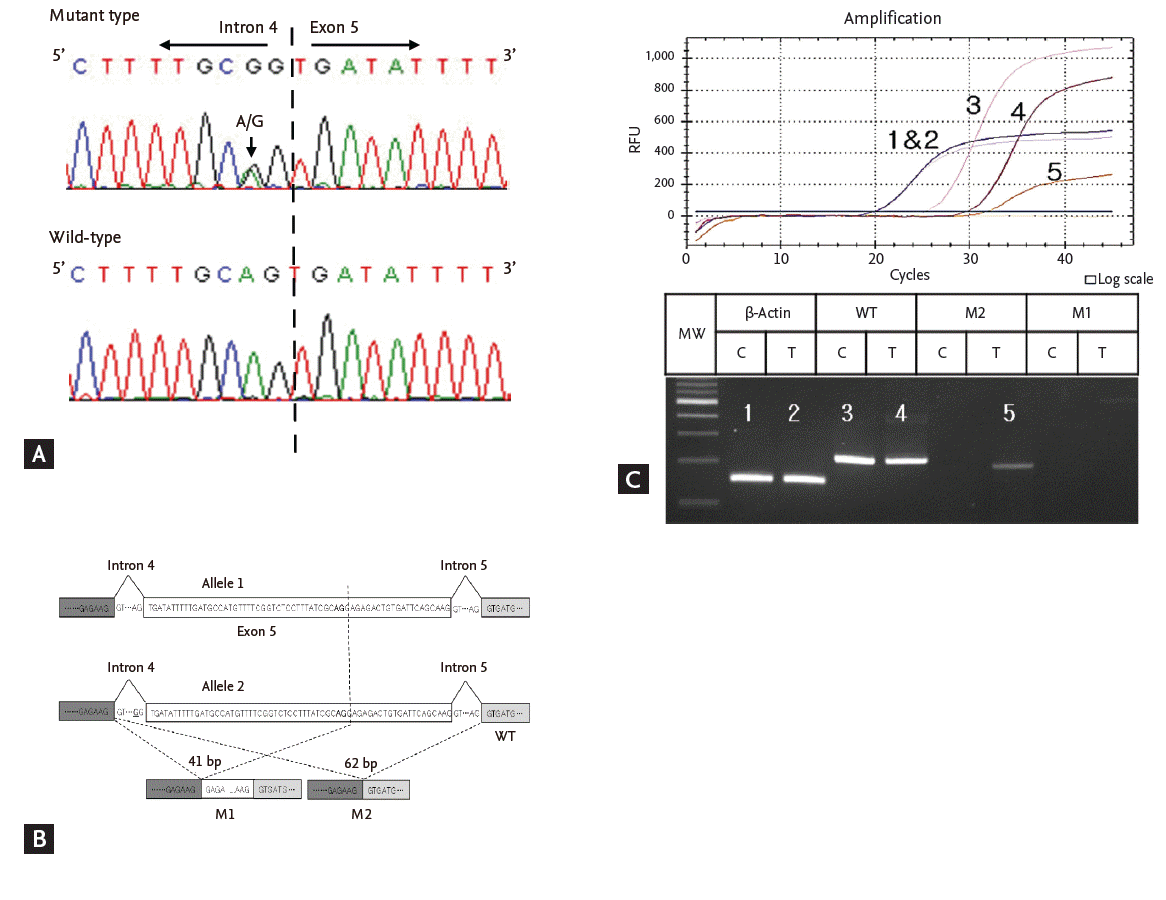
(A) Genomic DNA sequence analysis (5’) of the IVS4/exon 5 PRKAR1A splice site. The heterozygous IVS4A-2A>G mutation is in the second base of the 3’-acceptor splice site of intron 4A. The A→G substitution is indicated by the bold A/G. (B) Theoretically possible PRKAR1A transcripts resulting from the mutation IVS4A-2A>G: the wild-type (WT), 41-bp-deleted (M1), and 62-bp-deleted (M2) PRKAR1A mRNAs. The exonic splice acceptor site sequence in exon 4B is indicated by the bold AG. (C) Quantification of the differentially expressed PRKAR1A mRNAs in Carney complex. Plot of the real-time polymerase chain reaction (PCR) amplification and high-resolution gel electrophoresis for detection of PCR products generated during the amplification cycles. RFU, relative fluorescence unit; MW, molecular weight; C, normal adrenal tissue; T, adrenal tumor of Carney complex; WT, wild-type PRKAR1A mRNA; M1, PRKAR1A mRNA with a 41-bp deletion; M2, PRKAR1A mRNA with a 62-bp deletion.
The PRKAR1A gene is located on chromosome 17q22-24 and encodes the R1α regulatory subunit of cyclic adenosine monophosphate (cAMP)-dependent protein kinase A (PKA) that plays an important role in many signaling pathway [2]. Mutations of the PRKAR1A gene have been identified in 45% to 65% of patients with CNC [3] and CNC combined with PPNAD showed a higher rate (80%) of germline PRKAR1A mutations [4]. In the case of isolated PPNAD without a PRKAR1A mutation, another mutation was identified in the gene of the phosphodiesterase 11A (PDE11A) that regulated cAMP levels [3]. Most PRKAR1A mutations result in premature stop codons and mutant transcripts are degraded by nonsense-mediated mRNA decay, not showing mutant or shortened proteins [3]. The haploinsufficiency of wild-type PRKAR1A protein lead to increased PKA activity, followed by cell cycle abnormalities [5]. Only a minority (17.1%) of PRKAR1A mutation expressed mutant or shortened proteins with wild-type protein, showing more aggressive clinical manifestations [2].
Almost all PRKAR1A mutations in CNC are located in exons and the most frequent is a deletion in exon 5 [3], but we present a novel mutation of the PRKAR1A gene in intron 4. The test for an inactivating mutation of PRKAR1A is only a supplemental criterion for diagnosing CNC [1], but it can be useful to provide genetic counseling and monitor tumor growth in an asymptomatic CNC patient.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by the Catholic Medical Center Research Foundation in program year 2011.