An isolated cardiac relapse after allogeneic hematopoietic stem cell transplantation for acute lymphoblastic leukemia
Article information
To the Editor,
The incidence of extramedullary relapse (EMR) after allogeneic hematopoietic stem cell transplantation (allo-HSCT) for acute leukemia varies widely and ranges from 0.65% to over 20% [1]. Most cases of EMR after allo-HSCT occur with concomitant hematopoietic relapse. Isolated EMRs in acute leukemia patients who do not experience bone marrow relapse following allo-HSCT are relatively uncommon. However, isolated cardiac relapse after allo-HSCT is an exceedingly rare event. Here, we report a case of a 42-year-old male who presented with cardiac recurrence without concomitant marrow relapse after undergoing allo-HSCT from a matched sibling donor for B-cell acute lymphoblastic leukemia (ALL).
A 42-year-old Korean male presented in August 2013 with a 3-month history of exertional dyspnea and back pain. His total white blood cell count was 7.0 × 109/L, with 46% circulating blasts. His hemoglobin was 10.6 g/dL and platelet count was 337 × 109/L. Bone marrow sections showed a cellularity of 90%. The blasts accounted for up to 78% of bone marrow nucleated cells, which were found to be positive for CD10, CD13, CD19, CD22, CD33, CD34, and terminal deoxynucleotidyl transferase (Tdt) by flow cytometry. Cytogenetic analysis revealed a normal karyotype. A diagnosis of common B-cell ALL (French-American-British L1) was made. Complete remission (CR) was achieved after one induction chemotherapy composed of vincristine, daunorubicin, L-asparaginase, and prednisolone. Following CR, he was given two courses of consolidation therapy with intrathecal methotrexate. The first consolidation regimen was composed of daunorubicin, vincristine, prednisolone, and L-asparaginase, and the second of etoposide plus cytarabine. He received allo-HSCT from a matched sibling donor. The conditioning regimen consisted of cyclophosphamide (total 120 mg/kg in two doses) and busulfan (total 12.8 mg/kg in four doses). No abnormal findings were found in a routine pre-transplant workup that included laboratory studies, a chest radiograph, a pulmonary function test with the diffusing capacity of the lung for carbon monoxide, electrocardiogram, multigated acquisition scan, echocardiogram, and lumbar puncture with cytological examination. Cyclosporin and methotrexate were given for prophylaxis of graft-versus-host disease. A bone marrow biopsy performed 96 days after transplant confirmed trilineage regeneration and the presence of inv (9) (p12q13), which was a putative cytogenetic abnormality in the donor.
Seven months after allo-HSCT, he presented at the emergency department with sudden onset of dyspnea and chest discomfort. A 12-lead electrocardiogram (ECG), which had been within normal limits 7 months previously, showed a sinus rhythm with a new first degree atrioventricular block (P–R interval of 256 milliseconds) and T-wave inversion in precordial leads V4 to V6. Initial Troponin T and creatine kinase MB levels were within normal ranges, but were slightly elevated at 1.9 ng/mL (normal, < 1.5) and 10.6 ng/mL (normal, < 5.0), respectively, on the fourth day of hospitalization. Coronary computed tomography angiography revealed no significant coronary artery stenosis. An echocardiogram revealed a significant increase in overall left ventricular (LV) wall thickness, especially in the septal wall, which was associated with subclinical LV systolic dysfunction (Fig. 1). Cardiac magnetic resonance (CMR) showed diffuse multifocal infiltrative myocardial enhancement in the LV and right ventricular (RV) free wall (Fig. 2). To diagnose the cause of the abnormal myocardial thickening, a transvenous endomyocardial biopsy (EMB) was performed. Several hours after EMB, he developed a complete atrioventricular block but remained hemodynamically stable. He was taken to the catheterization laboratory, where a temporary transvenous pacing catheter was inserted. The EMB showed a normal endomyocardium. Because his condition was deteriorating rapidly and a pathologic diagnosis was critical for managing his condition, a surgical LV biopsy was performed under general anesthesia via a left mini-thoracotomy. The pathology revealed diffuse infiltration of atypical lymphocytes in the myocardium and pericardium. The cells had nuclei with vesicular chromatin and inconspicuous nucleoli and a scanty cytoplasm. Immunohistochemistry showed positive staining for CD10 and Tdt (Fig. 3). EMR was diagnosed. The total white blood cell count was 5.6 × 109/L with no circulating blasts, hemoglobin was 16.2 g/dL, and the platelet count was 64 × 109/L. Bone marrow examination revealed normocellular marrow with trilineage regeneration, but no evidence of marrow involvement with ALL blasts. Short tandem repeat polymerase chain reaction analysis revealed full donor chimerism. Chest, abdominal, and pelvic computed tomography scans showed no evidence of EMR.
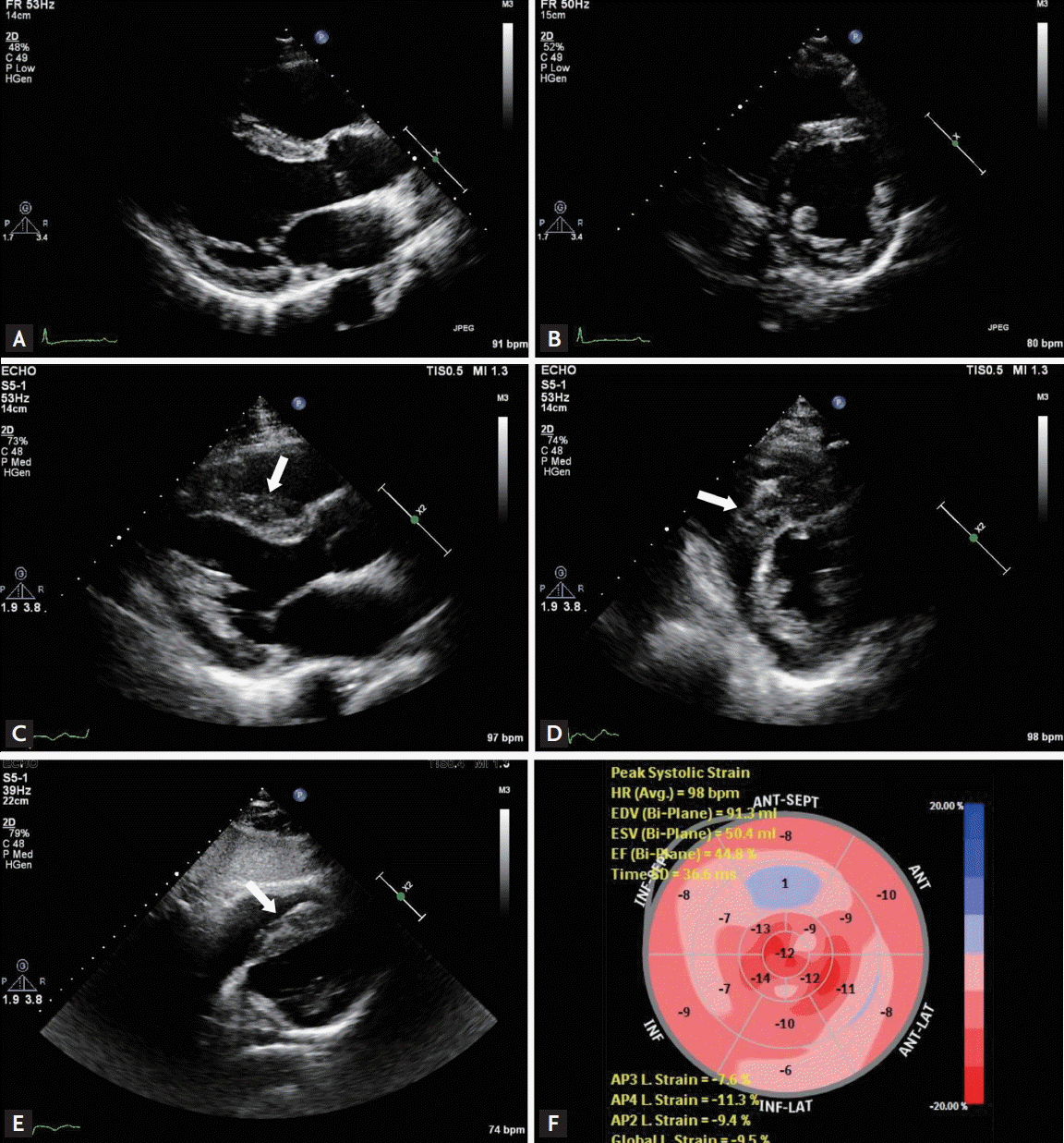
Echocardiographic findings. (A, B) Transthoracic echocardiography images performed 1 year ago showing normal left ventricular (LV) wall thickness and contractility. (C-E) At admission, overall LV wall thickness had increased significantly, especially in the septal wall (arrows). (F) Although the LV ejection fraction estimated using the modified Simpson’s method was 60%, the LV global longitudinal strain value was –9.5% (normal range, ≤ –16.0%), suggesting subclinical LV systolic dysfunction.

Cardiac magnetic resonance images. (A) T2-weighted short-tau inversion recovery images showing diffuse high-intensity signals in the left ventricle (LV) and right ventricular (RV) free wall. (B) Late-gadolinium enhancement images demonstrating multifocal infiltrative nodular and fuzzy myocardial enhancement in the LV and RV free wall.

Recurrent acute lymphoblastic leukemia (ALL) in the heart. (A) Ventricular biopsy showing diffuse infiltration of monotonous small cells in the myocardium and pericardium (H&E, ×40). (B) Lymphocytes showing infiltration and encroachment on cardiomyocytes. The atypical lymphocytes are medium-sized with round to oval nuclei showing vesicular chromatin and inconspicuous nucleoli, and have a scanty cytoplasm (high nuclear/cytoplasmic ratio; H&E, ×400). (C) Lymphocyte membranes expressing the pan-B-cell marker CD22 (×400). (D) Presence of terminal deoxynucleotidyl transferase in the nuclei of lymphocytes indicating their lymphoblastic nature (×400).
He received salvage chemotherapy composed of cytosine arabinoside and methotrexate. On his third day of salvage chemotherapy, he suddenly developed chest discomfort, a drowsy mentality, and hypotension. An ECG showed complete atrioventricular block with a ventricular rate of 49 beats per minute. A bedside echocardiogram revealed LV dysfunction with an estimated LV ejection fraction of 20% according to the modified Simpson’s method. Acute heart failure and complete atrioventricular block subsequently induced cardiopulmonary arrest despite transcutaneous cardiac pacing and intensive treatment with inotropic drugs. Cardiopulmonary resuscitation was performed, but the patient died from refractory cardiogenic shock.
After a single EMR of acute myeloblastic leukemia, ALL, chronic myeloid leukemia, or myelodysplastic syndrome, it is common to observe other EMRs within 1 year of the primary relapse [1]. Therefore, the early detection and adequate treatment of EMR is important to improve clinical outcomes of patients who have undergone allo-HSCT. Unfortunately, there has been no established strategy for EMR surveillance after HSCT [1]. For this reason, EMR is typically diagnosed only after the patient becomes symptomatic. Even more disturbing, secondary cardiac complications are more common than cardiac leukemic involvement in patients with acute leukemia [1]. Therefore, there is significant difficulty in establishing a diagnosis of isolated cardiac relapse.
The clinical manifestations of cardiac involvement of leukemic blasts are usually pericardial effusion, constrictive pericarditis and congestive heart failure [2]. There have also been occasional reports of cardiac leukemic involvement causing unusual presentations such as atrioventricular block or acute myocardial infarction [2]. In our current case, myocarditis was the suspected cause of myocardial thickening, LV dysfunction, high cardiac enzyme levels, and the complete atrioventricular block. However, CMR showed multifocal infiltrative nodular myocardial enhancement in the LV and RV free wall. To confirm abnormal heterogeneous myocardial infiltration with late gadolinium enhancement, an EMB was performed, but the biopsy showed a normal endomyocardium without abnormal cell infiltration. Considering the notoriously limited sensitivity of EMB, a surgical LV biopsy was performed instead of performing another EMB, which revealed diffuse infiltration of ALL blasts in the myocardium and pericardium. When patients with acute leukemia present with atrioventricular block or progressive heart failure after HSCT, consideration should be given not only to the acquired cardiac complications induced by cardiotoxic chemotherapeutic agent, infection, or graft versus host disease, but also to possible cardiac involvement of leukemic blasts.
One of the relatively common cardiac complications is chemotherapy-induced acute and chronic cardiac toxicity that usually manifests as cardiomyopathy, congestive heart failure, and pericarditis but can result in an alteration of cardiac rhythm, change in blood pressure, and acute myocardial infarction. Cardiotoxic chemotherapeutic agents can induce cardiomyopathy (chronic progressive cardiotoxicity) that shows diffuse myocardial fibrosis with a variable late gadolinium enhancement pattern on CMR [3]. In contrast, in our current patient, multifocal infiltrative nodular myocardial enhancement was identified by CMR. Considering this point, abnormal heterogeneous myocardial infiltration with late gadolinium enhancement on CMR may be the characteristic manifestation that can distinguish leukemic cardiac involvement from chemotherapy-induced cardiomyopathy.
To our knowledge, isolated cardiac relapse after HSCT for acute leukemia has only been reported in three patients previously [2,4,5]. Two of these patients had a bulky mediastinal mass at initial diagnosis of ALL. The pericardial relapse with infiltration of the myocardium and mediastinum in these two cases resulted from a failure in local eradication of blasts [2,4]. The other patient, a 33-year-old Caucasian male, who had relapsed with an isolated intracardiac mass, presented with right heart failure without a concomitant bone marrow relapse after receiving an allo-HSCT from a matched unrelated donor [5]. By contrast, our present patient experienced cardiac relapse as a result of diffuse myocardial infiltration of lymphoblastic cells. To the best of our knowledge, this is the first report of a case of isolated cardiac relapse resulting from diffuse infiltration of the myocardium following allo-HSCT for acute leukemia. Hence, unlike previous cases, our present patient was unique with respect to the use of a surgical cardiac biopsy for the diagnosis of cardiac relapse.
Notes
No potential conflict of interest relevant to this article was reported.