Impact of the β-1 adrenergic receptor polymorphism on tolerability and efficacy of bisoprolol therapy in Korean heart failure patients: association between β adrenergic receptor polymorphism and bisoprolol therapy in heart failure (ABBA) study
Article information
Abstract
Background/Aims:
We evaluated the association between coding region variants of adrenergic receptor genes and therapeutic effect in patients with congestive heart failure (CHF).
Methods:
One hundred patients with stable CHF (left ventricular ejection fraction [LVEF] < 45%) were enrolled. Enrolled patients started 1.25 mg bisoprolol treatment once daily, then up-titrated to the maximally tolerable dose, at which they were treated for 1 year.
Results:
Genotypic analysis was carried out, but the results were blinded to the investigators throughout the study period. At position 389 of the β-1 adrenergic receptor gene (ADRB1), the observed minor Gly allele frequency (Gly389Arg + Gly389Gly) was 0.21, and no deviation from Hardy-Weinberg equilibrium was observed in the genotypic distribution of Arg389Gly (p = 0.75). Heart rate was reduced from 80.8 ± 14.3 to 70.0 ± 15.0 beats per minute (p < 0.0001). There was no significant difference in final heart rate across genotypes. However, the Arg389Arg genotype group required significantly more bisoprolol compared to the Gly389X (Gly389Arg + Gly389Gly) group (5.26 ± 2.62 mg vs. 3.96 ± 2.05 mg, p = 0.022). There were no significant differences in LVEF changes or remodeling between two groups. Also, changes in exercise capacity and brain natriuretic peptide level were not significant. However, interestingly, there was a two-fold higher rate of readmission (21.2% vs. 10.0%, p = 0.162) and one CHF-related death in the Arg389Arg group.
Conclusions:
The ADRB1 Gly389X genotype showed greater response to bisoprolol than the Arg389Arg genotype, suggesting the potential of individually tailoring β-blocker therapy according to genotype.
INTRODUCTION
Pharmacogenomic effects continue to draw medical attention because they can substantially influence the therapeutic response. β-Blockers are a cornerstone treatment of congestive heart failure (CHF) [1-3]. Importantly, β-adrenergic receptor signaling genes are highly polymorphic, which substantially affects the incidence [4], prognosis [5-7], and therapeutic response of CHF. Among many single nucleotide polymorphisms (SNPs) in the β adrenergic receptor genes, Arg389Gly in the β-1 adrenergic receptor gene (ADRB1) and Arg16Gly and Glu27Gln in the β2-adrenergic receptor gene (ADRB2) have been widely investigated.
The ADRB1 variant encodes a Gly substitution for the highly conserved Arg389 within a region of the receptor that couples to intracellular signaling molecules [8]. In vitro, ADRB1 Arg389 increases its coupling to Gs compared with Gly389, increasing the activity of the β-1 adrenergic receptor [9]. In contrast, the Gly389 in ADRB1 reduced the receptor sensitivity as if it were partially blocked [10-13]. In the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) study, CHF patients with the Arg389Arg genotype were suggested to require a higher dose of β-blockade to achieve a treatment response similar to that of Gly carriers [14].
The ADRB2 polymorphism was mainly studied for its β-agonist therapeutic response in asthma patients. Similarly, ADBR2 Gly16 reduced β2-adrenergic receptor activity compared with Arg16 [15]. Although there has been no report showing variation in blood pressure (BP) or heart rate (HR) decrease in response to β-blocker therapy according to ADBR2 polymorphism [16], the ADRB2 Glu27 allele was reported to greatly improve systolic function in treatment with carvedilol, a nonspecific β-receptor blocker, compared with that of the Gln variant [17].
Remarkably, SNPs in ADRB1 and ADRB2 have distinct prevalence according to genetic background of the studied population. These interethnic differences might affect the clinical variability in β-blocker response [18]. African Americans have been shown to have a lower sensitivity to β-blocker therapy. In contrast, Chinese people have a higher sensitivity to β-blockers compared to other populations and show a stronger response to β-blocker therapy, requiring lower doses [19,20]. If genetic polymorphism exerts substantial impact on the pharmacologic response of β-blockers, individual dosing might be necessary to maximize the effect during CHF treatment. In this regard, this study aimed to investigate the association between ADRB1 polymorphism and therapeutic effect of bisoprolol in Korean patients with CHF.
METHODS
Study population
Chronic stable CHF patients of New York Heart Association (NYHA) class II to III with left ventricular ejection fraction (LVEF) ≤ 45% were recruited from 10 hospitals across Korea. Patients were excluded if they had one of the following conditions: decompensated CHF (NYHA class IV); acute myocardial infarction, unstable angina, coronary artery bypass graft, percutaneous coronary intervention, or valve surgery within 3 months; uncontrolled CHF due to hypertrophic obstructive cardiomyopathy; mild-to-severe valvular stenosis or severe (grade III/IV) valvular regurgitation; hypersensitivity to β-blockers; or systolic BP (SBP) < 90 mmHg at screening or HR < 55 beats per minute (bpm) based on a resting ECG at screening. Female patients with child bearing potential and pregnant or nursing women were not included in the study. The study was approved by the Institutional Review Board of each institute and the Korea Food and Drug Administration and was conducted in accordance with Korean Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent prior to participation. This study was registered at ClinicalTrials.gov (ID: NCT 01104558).
Bisoprolol dosing
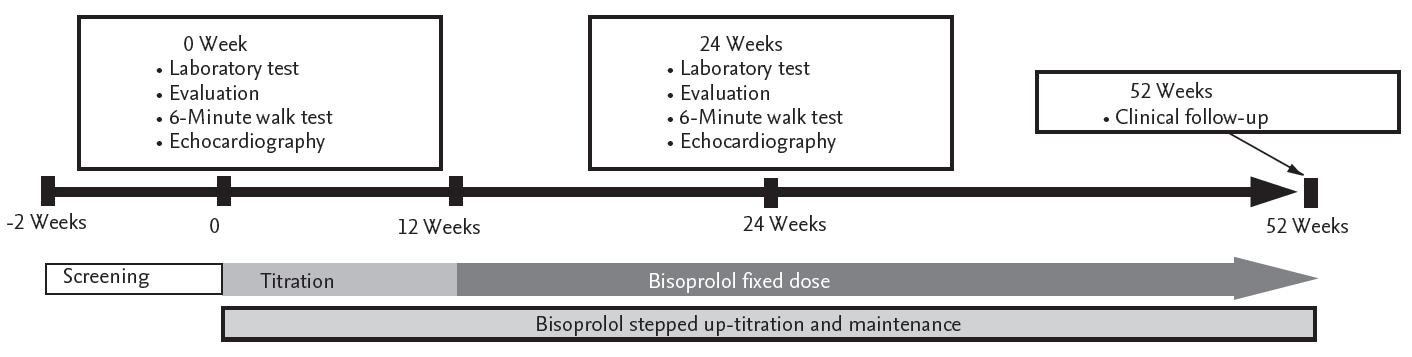
Patients were started with 1.25 mg bisoprolol treatment once a day; the dose was progressively up-titrated per 2-week interval to 2.5, 3.75, and 5 mg successively, taking into account the patient’s HR and tolerability based on subjective symptoms. The recommended maintenance dose of bisoprolol was 5 mg. However, if patients tolerated increased dosage, a maximum maintenance dose of 10 mg was recommended. After 12 weeks of up-titration, the bisoprolol doses were fixed and maintained throughout the study period. Standard CHF treatment and concomitant medications for various medical conditions were allowed at the investigator’s discretion. The study design is summarized in Fig. 1.
Genotyping
Patients were genotyped for ADRB1 Arg389Gly (rs1801253), ADRB2 Gly16Arg (rs1042713), and ADRB2 Gln27Glu (rs1042714). Cells in whole blood were lysed using a cell lysis solution. RNA was then removed using the RNA-digesting enzyme RNAse. Proteins were removed by protein precipitation solution. Finally, genomic DNA was recovered by precipitation in alcohol. A TaqMan SNP genotyping assay (5’ nuclease assay) was used for amplifying and detecting specific SNP alleles in the purified genomic DNA samples (1 to 20 ng) using real-time polymerase chain reaction system. Importantly, the patients and investigators were blinded to the genotypic results throughout the study period.
Clinical follow-up
BP, HR, and clinical events were monitored during the 52-week study period. Echocardiographic parameters such as LV dimension, LVEF, and E/A ratio were measured at baseline and 24 weeks after initiation of β-blocker therapy and then analyzed by two independent cardiologists who were blinded to patient information. The patients also underwent a 6-minute walk test at baseline and 24 weeks after the initiation of statin therapy.
Outcome
The primary endpoint was difference in LVEF after bisoprolol treatment. Secondary endpoints included change in 6-minute walk test, serum B-natriuretic peptide (BNP) level, BP, and HR level after bisoprolol treatment. Clinical endpoints included readmission and/or mortality throughout the study. For safety, adverse events, vital signs, and clinically significant abnormal values among the laboratory test results were evaluated [21].
Statistical analysis
As no previous data was available for the distribution pattern of the genetic polymorphisms of β-adrenergic receptor in Korean CHF patients, this study was initiated as an exploratory study with 100 patients. Statistical analysis was conducted using SAS 9.2 software (SAS Institute Inc., Cary, NC, USA). Results were expressed as mean, standard deviation, minimum, and maximum for baseline data including demographics, medical history, physical examination, and drug history. No adjustment was performed for baseline covariates. Changes in the efficacy parameters after bisoprolol therapy were evaluated within each genetic polymorphism by paired t test and two-sample t test. Variables with skewed distribution were log-transformed before analysis.
RESULTS
Study population
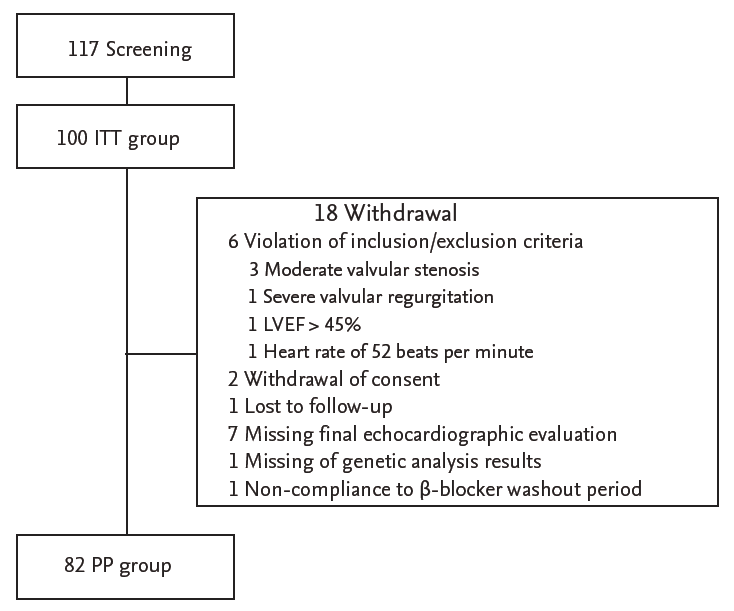
Of the 117 screened patients from 10 study institutions, 100 were prescribed bisoprolol after screening, 18 withdrew during the study, and 82 completed the study (Fig. 2). The majority of the patients were male (73%), and the mean age was 56.2 ± 13.2 years. NYHA class distribution was 97% in class II and 3% in class III. Genotyping was carried out in 83 patients. Baseline BP was 120.7 ± 21.7/78.1 ± 12.6 mmHg, and baseline HR was 80.8 ± 14.3 bpm. The baseline LVEF was 32.3% ± 8.0%, and the median BNP level at baseline was 820 pg/mL (25 and 75 percentile values of 228.2 and 1,177.5 pg/mL, respectively). Overall, 94% of patients were treated with renin-angiotensin system inhibitors (angiotensin converting enzyme inhibitor or angiotensin receptor blocker), and 34% of patients were treated with spironolactone. Of all patients, 51% were treated with digoxin, and 15% of patients were previously treated with β-blockers that were replaced with bisoprolol without a washing period.
β-Adrenergic receptor polymorphism
At position 389 of ADRB1, 53 patients were homozygous for the Arg genotype, five patients were homozygous for the Gly genotype, and 25 patients were heterozygous. The observed minor Gly allele frequency (Gly389Arg + Gly389Gly) was 0.21, and no deviation from Hardy-Weinberg equilibrium was observed in the genotype distribution of Arg389Gly (p = 0.75). The proportion of Gly carriers was lower than the reported proportion among blacks (range, 0.41 to 0.42) or whites (range, 0.27 to 0.28) [14,22]; however, the observed level was similar to the reported proportion among Japanese dilated cardiomyopathy patients (0.20) [23].
Both coding region variations of ADRB2 deviated from Hardy-Weinberg equilibrium and thus were excluded from further analyses. At position 16 of the β-2 adrenergic receptor (ADRB2), 14 patients were homozygous for the Arg genotype, 28 patients were homozygous for the Gly genotype, and 41 patients were heterozygous (p = 0.02). At position 27 of the β-2 adrenergic receptor, one patient was homozygous for the Glu genotype, 67 patients were homozygous for the Gln genotype, and 15 patients were heterozygous (p = 0.02).
Baseline characteristics according to ADRB1 polymorphism
Next, we compared the baseline characteristics of patients according to ADRB1 polymorphism. We merged the Gly389Arg group (n = 25) and the Gly389Gly group (n = 5) into the Gly389X group because the patient number in each group was far smaller than in the Agr389Arg group. The baseline characteristics of the patients between the two groups did not significantly differ except for a higher prevalence of diabetes mellitus (p = 0.023) in the Gly389X group (Table 1).
Tolerability to bisoprolol treatment according to genotype
Starting at a 1.25 mg dose once a day, the bisoprolol dose was up-titrated at 2-week intervals to 2.5, 3.75, and 5 mg and then to the maximal tolerated dose determined by the investigators. The final dose after 12 weeks was 4.79 ± 2.50 mg. There was no significant BP change from baseline to week 12; SBP changed from 120.7 ± 21.7 to 122.0 ± 19.7 mmHg (p = 0.571), while diastolic BP changed from 78.1 ± 12.6 to 76.4 ± 13.4 mmHg (p = 0.246), respectively. However, HR was reduced significantly from 80.8 ± 14.3 to 70.0 ± 15.0 bpm (p < 0.0001).
Interestingly, the ADRB1 Arg389Arg genotype group required a significantly larger amount of bisoprolol compared with the Gly389X (Gly389Arg + Gly389Gly) group (5.26 ± 2.62 mg vs. 3.96 ± 2.05 mg, p = 0.022) (Fig. 3A and 3B). Although the bisoprolol dose was higher in the ADRB1 Arg389Arg genotype group, there was no significant difference in HR reduction between the two groups (–8.9 ± 14.8 bpm vs. –11.9 ± 15.1 bpm, p = 0.43) (Fig. 3C).
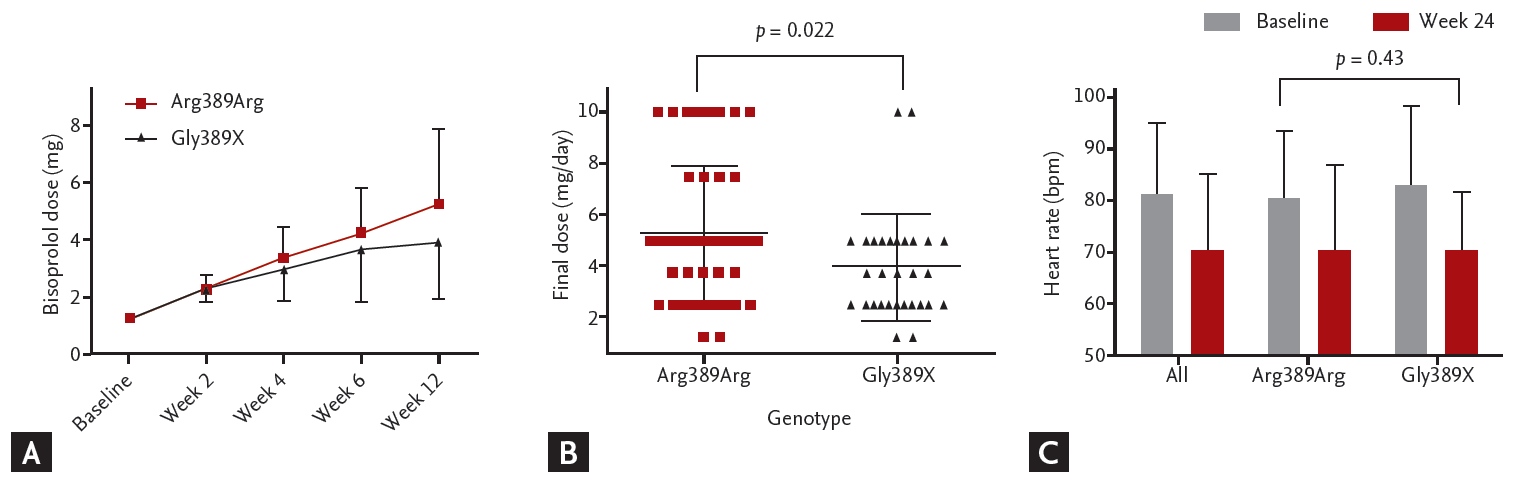
Bisoprolol response on heart rate according to β-1 adrenergic receptor gene 1 (ADRB1) genotype. (A) Up-titration curve of bisoprolol according to ADRB1 genotype. (B) Final bisoprolol dose after 12 weeks of up-titration. The p values are computed from two sample t test. (C) Heart rate change after 6 months of bisoprolol treatment according to ADRB1 genotype. The p values are computed from paired t test between baseline and week 24. bpm, beats per minute.
Left ventricular remodeling following bisoprolol treatment according to genotype
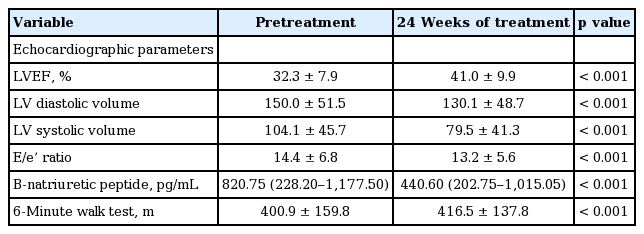
Following 24 weeks of bisoprolol therapy, LVEF was significantly improved from 32.3% ± 8.0% to 41.0% ± 9.9% (p < 0.0001). Left ventricular volume was also significantly reduced with end-systolic volume from 104.1 ± 45.7 to 79.5 ± 41.3 mL (p < 0.0001). Left ventricular end-diastolic pressure estimated by E/e’ wave ratio was also significantly decreased (p < 0.001) (Table 2).
There was no significant difference either in baseline or final LVEF between the ADRB1 Arg389Arg and Gly389X groups (Fig. 4A). Moreover, the reduction in left ventricular volume or end-diastolic pressure estimated by E/e’ wave ratio was not significantly different between the two genotype groups (Fig. 4B and 4C).

Left ventricular remodeling following bisoprolol treatment according to β-1 adrenergic receptor gene 1 (ADRB1) genotype. The p values are computed from paired t test between baseline and week 24. (A) Left ventricular ejection fraction (LVEF) change following bisoprolol treatment. (B) Left ventricular volume change following bisoprolol treatment. (C) Left ventricular E/e’ ratio change following bisoprolol treatment. LVEDV, left ventricular end-diastolic volume.
Short-term and long-term functional improvement according to genotype
Following 24 weeks of bisoprolol therapy, exercise capacity evaluated by 6-minute walk test was significantly improved from 401 ± 160 to 416 ± 138 m (p < 0.001) (Table 2). Moreover, there was no significant difference in change between the ADRB1 Arg389Arg genotype group and the Gly389X group (Fig. 5A)

Short-term and long-term functional improvements according to β-1 adrenergic receptor gene 1 (ADRB1) genotype. (A) Six-minute walk test before and 24 weeks after bisoprolol therapy. The p values are computed from paired t test between baseline and week 24. (B) B-natriuretic peptide (BNP) level before 24 weeks after bisoprolol therapy. As BNP level showed a skewed distribution, the results were compared after log-transformation. The p values are computed from paired t test between baseline and week 24. (C) Cardiovascular event for 1 year after bisoprolol treatment. The p values are computed from chi-square test. LogBNP, log-transformed B-natriuretic peptide.
Overall BNP level was also significantly reduced from 1,294.3 ± 2,679.0 to 987.0 ± 1,657.8 pg/mL (p = 0.023). Interestingly, BNP level only significantly decreased in the Gly389X group from 960.2 ± 783.9 to 581.2 ± 539.7 pg/mL (p = 0.005), whereas there was no significant change in the Arg389Arg group (1,510.8 ± 3,284.5 to 1,213.4 ± 2,001.2 pg/mL, p = 0.269) (LogBNP change in Fig. 5B).
Then, we investigated clinical events including readmission and mortality for 1 year. All patients continued bisoprolol treatment after the study. Although there was no statistical significance, a two-fold higher rate of readmission (21.2% vs. 10.0%, p = 0.162) and one CHF-related death were observed in the Arg389Arg group (Fig. 5C).
DISCUSSION
This study showed the substantial difference in response to β-blocker according to genotypic variations of adrenergic signaling genes. As no previous data was available for the distribution pattern of the genetic polymorphisms of β-adrenergic receptor in Korean CHF patients, we enrolled 100 patients with chronic stable CHF in an exploratory study. Although the number of patients was relatively small, the genetic distribution of ADRB1 Arg389Gly variation did not deviate from Hardy-Weinberg equilibrium. We utilized the β-blocker, bisoprolol, and the patients, echocardiographers, and investigators were blinded to the genotypic results throughout the study period, thereby minimizing the confounding effect. HR was significantly reduced by bisoprolol treatment. Although final HR was not significantly different between the genotypes, the patients in the Arg389Arg genotype group required a higher dose of bisoprolol compared with the Gly389X (Gly389Arg + Gly389Gly) group. This finding suggests that patients with the Arg389Arg genotype require a larger amount of β-blocker to achieve a similar HR reduction compared with other genotypes. In contrast, there was no significant BP change from baseline to week 12. Bisoprolol dose was up-titrated as scheduled, but other medication was modulated according to the investigators’ decision. Therefore, reduction in anti-hypertensive medication might result in the observed BP stability. There were no significant differences in changes in LVEF, exercise capacity, or BNP level change between the two groups. However, there was a two-fold higher rate of readmission and one CHF-related death in the Arg389Arg group.
ADRB1 is encoded by an intron-less gene located on chromosome 10q24-26, consisting of a short 5’ untranslated region of 86 base pairs (bp), an open reading frame that encodes a protein of 477 amino acids, and a 3’ untranslated region of about 900 bp [24]. The allele frequency of ADRB1 Gly389 among Asians was reported to be in the range of 0.20 to 0.30, which is quite lower than those in Caucasians (range, 0.24 to 0.34) and African Americans (range, 0.39 to 0.46) [23,25]. The allele frequency of ADRB1 Gly389 observed in this study was 0.21, which is similar to a previous report in Asians. ADRB1 Arg389 was reported to contribute to greater activity of β-1 adrenergic receptor compared to Gly389 [9,10,12,13]. In healthy individuals, HR responses to dobutamine, a β-1 agonist, were more than three-fold greater among ADRB1 Arg389 compared with Gly389 homozygotes [26]. As expected, we observed that an approximately 33% higher dose of bisoprolol was required to reduce the HR to the same range in the ADRB1 Arg389Arg group compared with in the Gly389X group. This finding has important clinical implication considering that it is often suggested that Asians have higher sensitivity to β-blockers compared to other populations, thereby requiring smaller doses [19,20]. In contrast, the allele frequency of ADRB1 Arg389 among Asians was even higher than in Caucasians and African Americans.
In various β-blocker trials of CHF patients, it has been strongly suggested that HR reduction is more important than β-blocker dose [27,28]. This suggestion is rational because the large difference in β-blocker efficacy according to genotype supports the importance of HR-guided therapy rather than the absolute drug dose itself.
Although the ADRB1 Arg389Gly genotype polymorphism is the most widely studied among various polymorphisms of adrenergic receptor genes, whether the Arg variant is protective or harmful is still controversial. Specifically, the genetic impact is different between epidemiologic and intervention studies with β-blockers. In the β-blocker ‘naïve’ state, the Gly389X genotype group was reported to have a better prognosis and greater survival [18,29]. In contrast, there was a report that suggested lack of benefit of the Gly389 allele [30]. Although Cresci et al. [7] suggested that the Arg389Arg genotype is protective in CHF progression, another report by Ogimoto et al. [23] reported that ADRB1 Arg389 allele carriers combined with deletion (range, 322 to 325) of the alpha2c receptor gene had more than a 10-fold higher risk of CHF. Conversely, homozygous Arg389 CHF individuals showed significantly better exercise capacity compared to homozygous Gly389 individuals [31] and were more sensitive to β-blockade, showing negative left ventricular remodeling [32,33] and better survival [7], reflecting comparatively better cardiac catecholamine signaling [8].
Remarkably, the results of previous studies suggest that the therapeutic impact of the ADRB1 genotype differs according to selectivity and dose of β-blockers [34]. The response to metoprolol, having high β-1 selective blocking activity, is better with the ADRB1 Arg389Arg genotype, resulting in improved LVEF and greater HR reduction [35,36]. Inhibition of the highly active ADRB1 Arg389Arg genotype is theoretically valuable in CHF [37]. In contrast, the ADRB1 Arg389Arg genotype combined with the ADRB2 Gln27 carrier, which was suggested to physiologically down-regulate β-2 adrenergic receptor, was reported to be associated with increased mortality in carvedilol, nonspecific β-1, and β-2 blocker treatment [37]. Similarly, CHF patients with the ADRB1 Arg389Arg genotype receiving low-dose (< 25 mg/daily) carvedilol showed a two-fold higher death risk compared with high-dose (> 25 mg/daily) users, which was not conferred in ADRB1 Gly carriers [14]. Lastly, bucindolol, which has a partial inverse β-agonistic effect, was reported to reduce mortality in ADRB1 Arg389Arg genotype CHF patients but had no clinical response in Gly389 carriers [10]. The reason for the beneficial response of the ADRB1 Arg389Arg genotype in advanced CHF patients might be due to the more sensitive and therefore less demanding, adrenergic agonist binding affinity [38].
In this study, although the extent of HR reduction was the same between the two genotype groups, BNP level was more highly decreased in the Gly389X genotype group than in Arg389Arg genotype group in spite higher β-blocker dose in the Arg389Arg group. Despite being statistically nonsignificant, the readmission rate was two-fold higher in the Arg389Arg group, and there was one heart failure-related death in this group. Together, these findings suggest a higher risk in the Arg389Arg genotype. This finding might be related to the relatively modest dose of β-blocker and HR reduction in this study resulting in the incomplete blockade of more active β-1 adrenergic receptor in the Arg389Arg genotype. Therefore, we cannot exclude the possibility that the results might be different if a higher dose and greater HR reduction were achieved in the Arg389Arg genotype.
The findings of our study should be considered in the context of several limitations. First, this study used a highly selective β-1 blocker, bisoprolol. Therefore, our findings may not apply to different types of β-blockers used to treat CHF patients. Second, although we adjusted for numerous predictors of left ventricular remodeling, the possibility of important unidentified confounding factors other than ADRB1 genotype cannot be excluded. Third, this study did not assess the effect of genotype on circulating markers of either sympathetic activation or the renin-angiotensin system. Fourth, the prevalence of diabetes mellitus was higher in the Gly389 genotype group. We did not evaluate the degree of diabetic autonomic dysfunction in the diabetic patients enrolled. If diabetic autonomic dysfunction was significant, HR response to β-blockers might be blunted [39]. However, the HR response to bisoprolol was greater in the ADRB1 Gly389X genotype, which suggests that diabetic autonomic neuropathy was negligible in this study. Lastly but most importantly, due to small sample size, we could not investigate the combined effects of multiple genotypes of the adrenergic receptor-related genes, the association of which was previously suggested [23].
In conclusion, in CHF patients, the ADRB1 Gly389X genotype showed greater response to bisoprolol than did the Arg389Arg genotype. The ADRB1 Gly389X genotype was also shown to have better prognosis than the Arg389Arg genotype. The pharmacogenomic effects observed in this study implicate the importance of individually tailoring β-blocker therapy.
KEY MESSAGE
1. β-Adrenergic receptor signaling genes are highly polymorphic, which substantially affects the incidence, prognosis, and therapeutic response of congestive heart failure (CHF).
2. As no previous data was available for the distribution pattern of the genetic polymorphisms of β-adrenergic receptor in Korean CHF patients, this study was initiated as an exploratory study with 100 patients.
3. The β-1 adrenergic receptor gene (ADRB1) Gly389X genotype showed greater response to bisoprolol than the Arg389Arg genotype, suggesting the potential of individually tailoring β-blocker therapy according to genotype.
Notes
This study was supported by a grant from Merck, Korea. The sponsor supported the supply of the investigational products, laboratory tests, and clinical research coordinator expenses.