Immediate multivessel revascularization may increase cardiac death and myocardial infarction in patients with ST-elevation myocardial infarction and multivessel coronary artery disease: data analysis from real world practice
Article information
Abstract
Background/Aims:
The best revascularization strategy for patients with both acute ST-elevation myocardial infarction (STEMI) and multivessel coronary disease (MVD) is still debatable. We aimed to compare the outcomes of multivessel revascularization (MVR) with those of culprit-only revascularization (COR).
Methods:
A cohort of 215 consecutive patients who had received primary angioplasty for STEMI and MVD were divided into two groups according to whether angioplasty had been also performed for a stenotic nonculprit artery. The primary endpoint was one-year major adverse cardiac events defined as a composite of cardiac death, recurrent myocardial infarction, or any repeat revascularization.
Results:
One-year major adverse cardiac events were not significantly different between MVR (n = 107) and COR (n = 108) groups. However, the one-year composite hard endpoint of cardiac death or recurrent myocardial infarction was notably increased in the MVR group compared to the COR group (20.0% vs. 8.9%, p = 0.024). In subgroup analysis, the hard endpoint was significantly more frequent in the immediate than in the staged MVR subgroup (26.6% vs. 9.8%, p = 0.036). The propensity score-matched cohorts confirmed these findings.
Conclusions:
In patients with STEMI and MVD, MVR, especially immediate MVR with primary percutaneous intervention, was not beneficial and led to worse outcomes. Therefore, we conclude that COR or staged MVR would be better strategies for patients with STEMI and MVD.
INTRODUCTION
Primary percutaneous coronary intervention (PCI) is the treatment of choice for reperfusion of patients with acute ST-elevation myocardial infarction (STEMI) [1]. Among patients with STEMI who undergo primary PCI, up to 65% have multivessel coronary disease (MVD) and suffer from increased morbidity and mortality [2,3]. For patients with both STEMI and MVD, current guidelines recommend that PCI be performed only in the culprit artery at the time of primary PCI unless they are hemodynamically compromised [4-7]. Despite considerable advances in PCI devices and physicians’ skill and the explosive increase in the number of primary PCIs performed in patients with STEMI, multivessel revascularization (MVR) still remains challenging in real world practice.
Results and recommendations are conflicting; some authors reported that MVR was feasible and safe [8-11]; others stated that in patients with MVD, primary PCI should be culprit-only revascularization (COR) [12]. Recently, the large randomized PRAMI (PReventive Angioplasty in Myocardial Infarction) trial found MVR was beneficial in terms of cardiac death and nonfatal myocardial infarction (MI) as well as refractory angina or repeat revascularization [13]. However, this study compared an immediate PCI strategy for nonculprit lesions with conservative strategy; the immediate strategy used an extremely strict strategy that did not permit a staged procedure. In the conservative group, only two patients among 231 patients received PCI for nonculprit lesions.
Concerns remain about whether to treat nonculprit lesions in patients with acute STEMI and when to treat, immediately or staged. Therefore, the purpose of the present study is to compare the efficacy and safety of MVR with COR and immediate with staged MVR in patients with STEMI and MVD.
METHODS
Study population
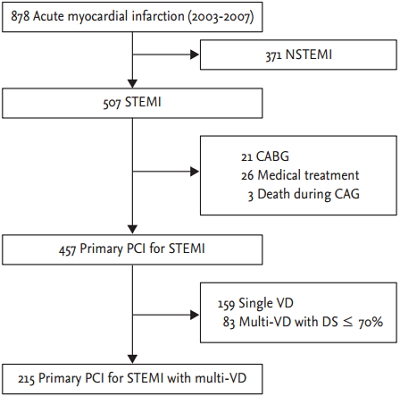
Two hundred fifteen consecutive patients with STEMI and MVD who underwent successful primary PCI in either the culprit artery only or culprit and nonculprit arteries in Seoul National University Bundang Hospital between July 2003 and July 2007 were enrolled (Fig. 1). Acute STEMI was defined as acute chest pain for more than 20 minutes within 12 hours of onset and electrocardiograms showing either an ST-segment elevation greater than 0.1 mV in two or more contiguous leads or a new left bundle branch block. MVD was defined as diameter stenosis of more than 70% estimated visually in two or more major epicardial coronary arteries, including the infarct-related artery. Patients with PCI that was unsuccessful, based on angiography, or with contraindication to the administration of aspirin, heparin or clopidogrel, were excluded. Patients with cardiogenic shock at admission, or a previous history of PCI, coronary artery bypass graft surgery or MI were all included.

Patient selection flow diagram. NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; CABG, coronary artery bypass grafting; CAG, coronary angiography; PCI, percutaneous coronary intervention; VD, vessel disease; DS, diameter stenosis.
Patients were categorized into two groups, the MVR and COR group, according to the PCIs performed. In the COR group, patients underwent PCI only in the culprit artery, and nonculprit arteries remained untreated until discharge. Nonculprit arteries were revascularized based on clinical presentation during follow-up. In the MVR group, patients underwent PCI in the culprit and nonculprit arteries, either immediately at the time of the primary PCI or as a staged procedure 3 or 4 days after primary PCI.
The study protocol was approved by the Institutional Review Board and all patients submitted written informed consent, as specified by the ethical guidelines of Declaration of Helsinki, 2008.
Coronary angiography and PCI procedure
All patients received 300 mg of aspirin (chewed) in the emergency room and 300 to 600 mg of clopidogrel in the emergency room or cardiac catheterization room prior to the PCI. In the catheterization room just before the PCI, a bolus of 100 IU/kg unfractionated heparin was injected intravenously, and additional heparin was administered, if necessary, to maintain an activated coagulation time between 250 and 350 seconds.
Angiography and PCI were performed in the standard manner. After primary PCI was completed, the decision of whether to perform PCI in stenotic (> 70%) nonculprit arteries was made by the on-duty interventional cardiologist. Platelet glycoprotein IIb/IIIa receptor antagonist was given at the operator’s discretion.
During hospitalization, cardiac enzyme levels were measured every 6 hours for the first 48 hours and then daily until discharge. Transthoracic echocardiography was performed 3 or 4 days after admission.
Procedural success was defined as Thrombolysis in Myocardial Infarction (TIMI) flow grade III with final stenosis less than 20% without death, recurrent MI, or emergent coronary artery bypass graft surgery.
Primary and secondary outcomes
The primary endpoint was 1-year major adverse cardiac events (MACEs), defined as a composite of cardiac death, recurrent MI, or any repeat revascularization. Any repeat revascularization included culprit artery target vessel revascularization (TVR), nonculprit artery target lesion revascularization, or nonculprit artery nontarget lesion revascularization. Nonculprit artery target lesion revascularization was in lesions that underwent PCI in nonculprit arteries during index hospitalization and nonculprit artery nontarget lesion revascularization was in lesions in nonculprit arteries that were not treated with PCI initially. Revascularization during the follow-up period, whether it was for previous target lesions or not, was performed if physicians believed that those lesions were responsible for recurrent angina or myocardial ischemia that had progressed angiographically even without angina, based on noninvasive or invasive study.
Recurrent MI was defined as cardiac troponin or creatinine kinase-MB isoenzyme (CK-MB) levels greater than three times the upper normal limit in association with ischemic chest pain or new ischemic electrocardiographic findings after normalization from an initial increase in the index STEMI [14]. It included periprocedural MI as well as spontaneous MI during follow-up period after the primary PCI.
Secondary endpoints were cardiac death, recurrent MI, the hard endpoint of cardiac death or recurrent MI as a composite, or any repeat revascularization during the 1-year follow-up period. Patient follow-up data were obtained primarily from medical records. For a few patients, who did not visit an outpatient clinic during the 1-year follow-up period, data were obtained from telephone interviews.
Statistical analysis
All values were expressed as mean ± standard deviation for continuous variables and as percentage for categorical variables. The baseline characteristics of the two groups were compared using Student t test for continuous variables and chi-square test for categorical variables. The cumulative incidences of events were calculated using the Kaplan-Meier method and were compared using the log-rank test. Cox regression analyses were applied to examine predictors of events. Multivariate logistic models included gender, age, and variables that were associated with outcomes based on a univariate analysis p value of < 0.1. The strengths of the association with survival were presented as hazard ratios and 95% confidence interval (CI).
Because the comparison groups were assigned to treatment non-randomly, to reduce selection bias, a 1:1 matched analysis without replacement was performed using a propensity score. The propensity score was generated using logistic regression multivariate modeling. Propensity score matching based on the TIMI risk score for STEMI was applied. To balance the two treatment groups in terms of clinical risk in STEMI, the individual components of the TIMI risk score was used to calculate the propensity score. The TIMI risk score is a 14-point scale that includes eight factors: age ≥ 75 or 65 to 74 (3 or 2 points); history of diabetes, hypertension, or angina (1 point); systolic blood pressure < 100 mmHg (3 points); heart rate > 100/min (2 points); Killip class II to IV (2 points); weight < 67 kg (1 point); anterior ST elevation or left bundle-branch block (1 point); and time to reperfusion therapy > 4 hours (1 point) [15]. Nearest neighbor matching with a caliper width of 0.2 standard deviation was used because this value has been shown to eliminate over 90% of the bias in the observed confounders [16]. Baseline clinical characteristics and the 1-year outcomes of the propensity score matched group were compared using paired t test for continuous variables or McNemar test for categorical variables.
All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA), R programming language version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria) and a p < 0.05 was considered statistically significant.
RESULTS
Baseline clinical and procedural characteristics
The MVR and COR groups included 107 and 108 patients, respectively. The enrolled patients had a mean age of 63.6 ± 13.0 years and 73% were male. The baseline clinical characteristics of the MVR and COR groups did not differ, except for a slightly higher peak CK-MB in the COR group. Based on angiography, PCI was performed in a mean of 2.3 and 1.1 lesions in the MVR and COR groups, respectively. The mean procedure time was 15 minutes longer in the MVR group. Intra-aortic counterpulsation balloon pump and glycoprotein IIb/IIIa receptor antagonist were used with similar frequency in the two groups.
Drug-eluting stents (DES) were implanted in 175 patients, bare metal stents were implanted in 24 patients, and both DES and bare metal stents were implanted in 15 patients. Balloon angioplasty was performed in only one patient. Culprit lesion stents diameter and the total stent length were not different between the groups (Table 1). In the MVR group, only 20 patients were treated based on noninvasive assessment of ischemia of the nonculprit lesions.
In-hospital and 6-month outcomes
The median duration of hospitalization was 7 days in the MVR group and 6 days in the COR group; this difference was not significant. The composite hard endpoint of cardiac death or recurrent MI had a tendency toward greater frequency in the MVR group (11.2% vs. 5.6%, p = 0.13). The 6-month left ventricular ejection fraction did not differ between the two groups (52.4% ± 11.8% in the MVR group vs. 50.5% ± 11.3% in the COR group, p = 0.31). The 6-month N-terminal-pro B-type natriuretic peptide level was also similar (434 ± 1,132 pg/mL in the MVR group vs. 418 ± 608 pg/mL in the COR group, p = 0.92). The normal range of N-terminal pro-B-type natriuretic peptide level was defined as < 0.88 pg/mL.
One-year clinical outcomes
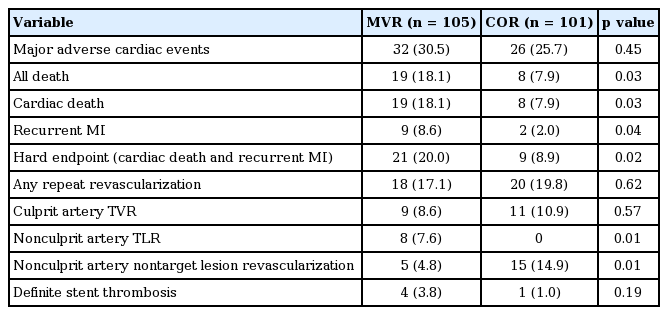
Two patients in the MVR group and seven patients in the COR group were lost to 1-year follow-up. Thus, 1-year clinical follow-up rates were 98.1% and 93.5%, respectively. The frequency of 1-year MACE (composite of cardiac death, recurrent MI, or any repeat revascularization), the primary outcome, did not differ significantly between the two groups (30.5% vs. 25.7%, p = 0.45). However, the hard endpoint composite, one of the secondary outcomes, was significantly more frequent in the MVR group than in the COR group (20.0% vs. 8.9%, p = 0.024). Each component of the hard endpoint, cardiac death and recurrent MI, also occurred more frequently in MVR group. The frequency of any repeat revascularization and culprit artery TVR were not significantly different between the groups (17.1% vs. 19.8%, p = 0.62; 8.6% vs. 10.9%, p = 0.57, respectively). Among 38 patients who underwent any repeat revascularization, functional studies were performed in six patients (15.8%). In five patients, myocardial SPECT (single-photon emission computed tomography) was performed, and in one patient, fractional flow reserve (FFR)-guided PCI was performed. The frequency of repeat nonculprit artery revascularization, including target lesions and nontarget lesions, was also similar (12.4% vs. 14.9%, p = 0.61). Among 18 repeat revascularization procedures performed in the MVR group, eight were in nonculprit artery target lesions, which was comparable to the frequency of culprit artery TVR (nine cases). In the COR group, nonculprit artery nontarget lesion revascularization was performed in only 14.9% of patients (Table 2).
In the MVR group, PCI of nonculprit arteries was performed either immediately or as a staged procedure during the index hospitalization. The MVR group was categorized into two subgroups according to the timing of the PCI in nonculprit arteries during the index hospitalization. Subgroup analysis compared immediate (n = 66) and staged (n = 41) MVR to determine which subgroup contributed more to the worse outcome experienced in the entire MVR group.
Baseline characteristics of the immediate and staged MVR subgroups were similar. In-hospital MACE and the hard endpoint were more common in the immediate MVR subgroup, although not significantly different (both MACE and hard endpoint, 15.2% vs. 4.9%, p = 0.10).
Among the 1-year outcomes, the hard endpoint occurred significantly more often in the immediate MVR subgroup than in the staged MVR subgroup (Table 3). This difference was mainly attributable to greater 1-year cardiac mortality in the immediate MVR subgroup (25.0% vs. 7.3%, p = 0.02).

Subgroup analysis: baseline characteristics and outcomes of patients with immediate versus staged MVR
Survival analysis showed that 1-year MACE-free survival was not significantly different between the COR and MVR groups (Fig. 2A); between the COR group and immediate MVR subgroup; or between the COR group and staged MVR subgroup (Fig. 3A). However, 1-year hard endpoint-free survival was significantly lower in the MVR group than in the COR group (Fig. 2B). This difference was explained primarily by the lower hard endpoint-free survival of the immediate MVR subgroup compared with the staged MVR subgroup (Fig. 3B). In our institution, 6-month follow-up angiography was recommended to all patients, and repeat revascularization was performed if clinically indicated. The superior MACE-free survival of the COR group appears to decline at approximately 6 months. Follow-up angiography was performed at 6 months in 73.8% and 72.2% (p = 0.79) of the MVR and COR groups, respectively.
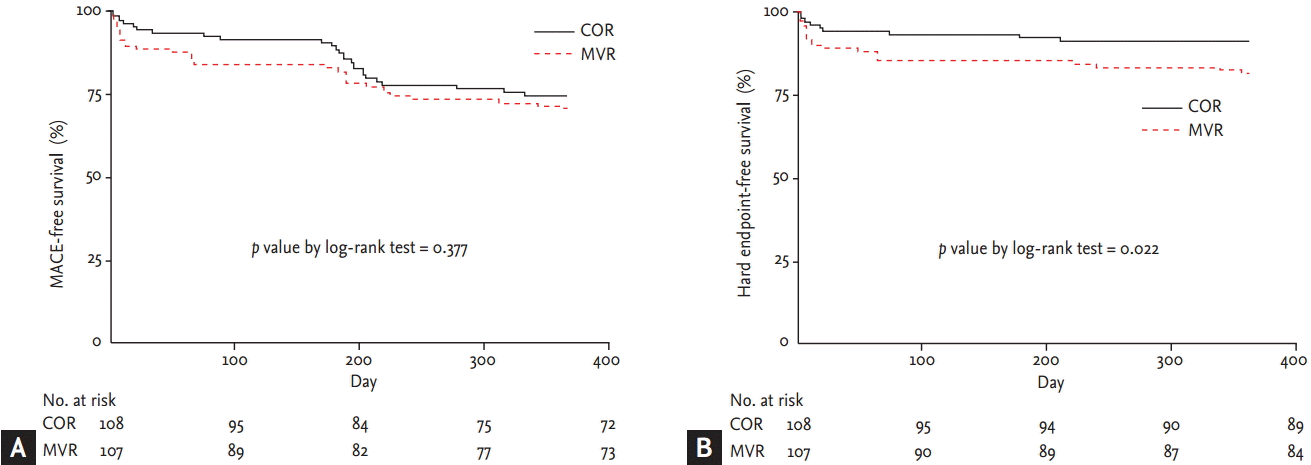
Comparison of 1-year event-free survival curves between multivessel revascularization (MVR) and culprit-only revascularization (COR) groups, Kaplan-Meier method. (A) The 1-year major adverse cardiac events (MACE)-free survival was not significantly different between the groups. (B) However, the survival free from the hard endpoint of cardiac death or recurrent myocardial infarction was significantly lower in the MVR group than in the COR group.
Comparison of the 1-year event-free survival curves among culprit-only revascularization (COR), immediate and staged multivessel revascularization (MVR) subgroups, Kaplan-Meier method. (A) One-year major adverse cardiac events (MACE)-free survival was not significantly different among the three groups. (B) However, hard endpoint-free survival of the immediate MVR subgroup was significantly lower than that of the staged MVR subgroup. The staged MVR subgroup had hard endpoint-free survival similar to the COR group.
Predictors of the hard endpoint
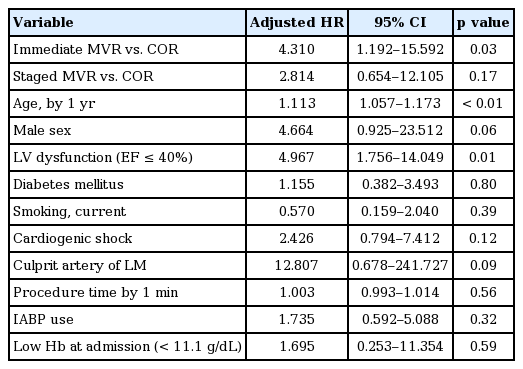
Based on univariate regression analysis, the immediate MVR group appeared to be one of the significant predictors of the hard endpoint (hazard ratio, 3.145; 95% CI, 1.390 to 7.121). Other determinants of the hard endpoint were left ventricular dysfunction (ejection fraction ≤ 40%), cardiogenic shock, culprit artery located in the left main trunk, duration of the procedure, use of intra-aortic counterpulsation balloon pump, and low hemoglobin level at admission. Multivariate analysis, adjusted for the likely predictors identified in univariate analysis, found immediate MVR, left ventricular dysfunction, and age to be independent predictors of the hard endpoint (Table 4).
Causes of deaths
In the COR group, heart failure was the most common cause of in-hospital death, which could explain three of the six deaths. There were one in-hospital fatal stent thrombosis and one sudden cardiac death during follow-up. In contrast, in the MVR group, and especially in the immediate MVR subgroup, definite stent thrombosis and cardiogenic shock were the prominent causes of in-hospital death, which occurred in three of 10 deaths. Among three definite stent thrombotic deaths in the immediate MVR subgroup, one was an acute stent thrombosis that occurred immediately after primary PCI, and the other two deaths were due to subacute stent thromboses. During the period between discharge and the 1-year follow-up, sudden death was the most remarkable cause of death in the immediate MVR subgroup (three of six deaths). However, there was no significant difference in cause of death between the MVR and COR groups or between the immediate and staged MVR subgroups.
Outcomes of the propensity score-matched cohorts
To control potential differences in baseline characteristics between the two groups in this non-randomized study, we constructed cohorts matched on a propensity score using the variables in the TIMI risk score for STEMI. This yielded 90 matched pairs of patients for the two treatment groups; the baseline characteristics of the matched pairs were comparable. At 1-year, there was no significant difference in the incidence of MACE between the two groups. However, the incidence of the hard endpoint, either cardiac death or recurrent MI, was still significantly higher in the MVR group than in the COR group (18.0% vs. 7.9%, p = 0.044) (Table 5).
DISCUSSION
The main findings of this study are that in patients treated with primary PCI, MVR did not decrease MACE and was associated with higher rates of cardiac death, recurrent MI than in COR, and immediate MVR was the main predictor of the poorer outcome in the MVR group, based on multivariate analysis. Therefore, the current study does not support MVR, especially immediate MVR, as a strategy for primary PCI.
MVR in STEMI patients is supported by several reports of the importance of noninfarct zone function, nonculprit artery flow, and multiple coronary plaques in patients with acute MI. Grines et al. [17] reported that in-hospital mortality was closely related to function in the noninfarct zone, and that the absence of MVD was the clinical factor most strongly associated with enhanced function in the noninfarct zone. In another report, Gibson et al. [18] showed that in patients with acute MI, nonculprit artery flow was 45% slower than normal flow and that the presence of nonculprit artery stenosis was one of the factors associated with slower nonculprit artery flow. Moreover, Goldstein et al. [19] reported that multiple coronary plaques were observed in 40% of patients with acute MI and were associated with adverse clinical outcomes. Thus, the presence of additional atherosclerotic plaques may contribute to future cardiovascular events.
Early studies of MVR were promising. One single-arm study showed a 95% to 97% success rate and acceptable rates of 2-year survival or MACE [20]. In their study of 120 patients with MI and MVD, Qarawani et al. [10] revealed that the in-hospital composite endpoint, recurrent ischemia, reinfarction, acute heart failure, or mortality, occurred significantly less often in the MVR group than in the COR group. However, the treatment group sizes were unbalanced (95 in the MVR group and 25 in the COR group), and the benefit was limited to in-hospital outcomes. In their study, a tendency toward greater 1-year mortality was found in the MVR group (9.4% in the MVR group vs. 8% in the COR group, p = 0.06).
Politi et al. [11] randomly allocated 214 patients to immediate MVR, staged MVR, or COR groups and reported that the COR group had worse outcomes than did the immediate or staged MVR groups in terms of re-hospitalization, repeat revascularization, and repeated PCI. However, the patient numbers were not well balanced among the three groups despite random allocation.
Although a few reports have shown no benefit from MVR [8,9,21] or supported COR [12,22,23], some possibility of reducing MACE with MVR remains because the use of DES can markedly decrease repeated procedure without affecting mortality. The present study revealed that the unfavorable outcomes of MVR in STEMI patients persisted, even in the DES era. No significant differences in the 1-year MACE were observed between MVR and COR groups in the present study. These findings were consistent after propensity score matching.
It is necessary to discuss why the MVR group experienced the hard endpoint more often in this study. The finding of worse outcomes in the immediate MVR subgroup is relevant to this question. Only two previous studies compared the outcomes of immediate MVR, staged MVR, and COR groups. In one of them, patients undergoing immediate MVR had a higher mortality rate at both 30 days and 1 year [12]. In the other, the immediate and staged MVR groups experienced similar frequencies of cardiac death, reinfarction, and repeat revascularization [11]. However, in the later study, the patient population had relatively less risk, glycoprotein IIb/IIIa receptor antagonist was used more frequently, and implantation of DES, which could be potentially more thrombogenic in MI patients, was used less often than in our study. Notably, the immediate MVR subgroup was an independent poor prognostic factor after potential confounders were controlled by multivariate analysis. Our finding that fatal stent thrombosis and sudden cardiac death (three cases of each) occurred more frequently in the immediate MVR subgroup than in the COR group (one case of each) suggests that stent thrombosis could be a cause of the more frequent hard endpoint observed in the immediate MVR subgroup. In the clinical setting of primary PCI, mechanical factors that might favor stent thrombosis (such as, stent underexpansion, inadequate lesion preparation, and incomplete apposition) are remarkably more likely to occur than with conventional procedures and are directly related to the number of inserted stents. Although the PRAMI trial revealed that immediate MVR could be better than COR for acute STEMI, it neither reported the illness severity of the study groups nor compared immediate MVR with staged MVR, which might be a safer and more effective strategy.
It is not surprising MVR did not result in any clinical benefit for the hard endpoint in the present study considering the results of the Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME) trial and Ntalianis et al.’s study [24-26], which showed FFR-guided PCI was also reliable in nonculprit lesions during the acute phase of acute coronary syndrome. The study proved that angiography-guided PCI was not beneficial in patients with multivessel disease in terms of repeat revascularization as well as cardiac death and MI. In the present study, the majority of patients in the MVR group underwent angiography-guided PCI for their nonculprit artery, so our results are consistent with the FAME study, if confined to the strategy for noninfarcted multivessel disease. A portion of the nonculprit lesions that were revascularized may not be responsible for ischemia. Tonino et al. [27] showed that 46% of the angiography-guided decisions incorrectly predicted ischemia. For these nonischemic lesions, PCI can produce worse outcomes than optimal medical therapy alone, according to Legalery et al.’s findings [28]. Similarly, revascularization of nonculprit lesions that lack evidence of ischemia in STEMI patients might be unnecessary and contribute to poorer results in the MVR group, such as more frequent hard endpoints observed in the present study. In the setting of primary PCI for STEMI patients with MVD except for cardiogenic shock, it is now evident that in nonculprit lesions ‘to leave it alone’ is currently a better strategy than ‘to do something.’
Our study differs from previous studies regarding propensity score matching. Propensity scores have been used to reduce bias in observational studies in many fields and is widely used in cardiovascular research [29]. Our cohorts were matched on their TIMI risk score. The TIMI risk score is a risk scoring system for predicting mortality in patients with STEMI, represents the sum of independent predictors of mortality, is based on the result of the Intravenous nPA for the Treatment of Infarcting Myocardium Early II trial [15] and was well validated in large-scale registries that included patients treated with pharmacological reperfusion or primary angioplasty [30-32].
There are a few limitations in this study. First, this study used a retrospective cohort design and the treatment was non-randomized. The assignment to MVR or COR was solely determined by the on-duty interventional cardiologist. Therefore, it is still possible that higher risk patients underwent MVR although we tried to overcome this intrinsic limitation by propensity score matching.
Second, the lack of myocardial ischemia assessment for nonculprit arteries might be another shortcoming of this study. However, there is no well-standardized method for assessing ischemia of nonculprit lesions during primary PCI in patients with STEMI. That is also a reason why we should be cautious about treatment of nonculprit arteries in STEMI.
Third, how many patients in each group became completely revascularized was not evaluated. In STEMI patients with multivessel disease, complete revascularization may result in a benefit [33]. However, in our study it is unlikely that non-revascularized lesions affected the hard endpoint unfavorably only in MVR group because comparable lesions in the COR group were also left non-revascularized.
Fourth, the relatively high frequency of angiography, performed at 6 months of follow-up, could bias the MACE, especially repeat revascularization. However, this bias is unlikely, because the two groups had similar frequencies of follow-up angiography and because repeat intervention was guided by symptoms or signs of ischemia not by the oculostenotic reflex.
KEY MESSAGE
1. In patients with ST-elevation myocardial infarction (STEMI) and multivessel coronary disease (MVD), multivessel revascularization (MVR) was not beneficial regarding major adverse cardiac event and was worse than culprit-only revascularization (COR) in terms of cardiac death and recurrent myocardial infarction during 1-year follow-up.
2. COR or staged MVR would be better treatment options than immediate MVR for patients with STEMI and MVD undergoing primary percutaneous coronary intervention.
Notes
No potential conflict of interest relevant to this article was reported.