Functional dyspepsia: new insights into pathogenesis and therapy
Article information
Abstract
One in 10 people suffer from functional dyspepsia (FD), a clinical syndrome comprising chronic bothersome early satiety, or postprandial fullness, or epigastric pain or burning. Postprandial distress syndrome (PDS, comprising early satiety and/or postprandial fullness) and epigastric pain syndrome (EPS) are increasingly accepted as valid clinical entities, based on new insights into the pathophysiology and the results of clinical trials. Diagnosis is based on the clinical history, and exclusion of peptic ulcer and cancer by endoscopy. Evidence is accumulating FD and gastroesophageal ref lux disease are part of the same disease spectrum in a major subset. The causes of FD remain to be established, but accumulating data suggest infections and possibly food may play an important role in subsets. FD does not equate with no pathology; duodenal eosinophilia is now an accepted association, and Helicobacter pylori infection is considered to be causally linked to dyspepsia although only a minority will respond to eradication. In those with EPS, acid suppression therapy is a first line therapy; consider a H2 blocker even if proton pump inhibitor fails. In PDS, a prokinetic is preferred. Second line therapy includes administration of a tricyclic antidepressant in low doses, or mirtazapine, but not a selective serotonin reuptake inhibitor.
INTRODUCTION
Excessive fullness after eating or the inability to finish a normal sized meal and recurrent epigastric pain are common symptoms and reasons for consulting a medical professional [1]. Structural investigations, including esophagogastroduodenoscopy (EGD), usually fail to identify an obvious organic explanation, and these patients are labeled as having functional dyspepsia (FD). Other terms applied to the same condition in the past have included nervous dyspepsia, non-ulcer dyspepsia (NUD) and essential dyspepsia, but FD is now the most common diagnosis and is included in the Rome criteria classification of functional gastrointestinal disorders (FGIDs). FD is important because it is not only highly prevalent but also impairs quality of life, work performance and family relationships and incurs a high healthcare cost worldwide [2].
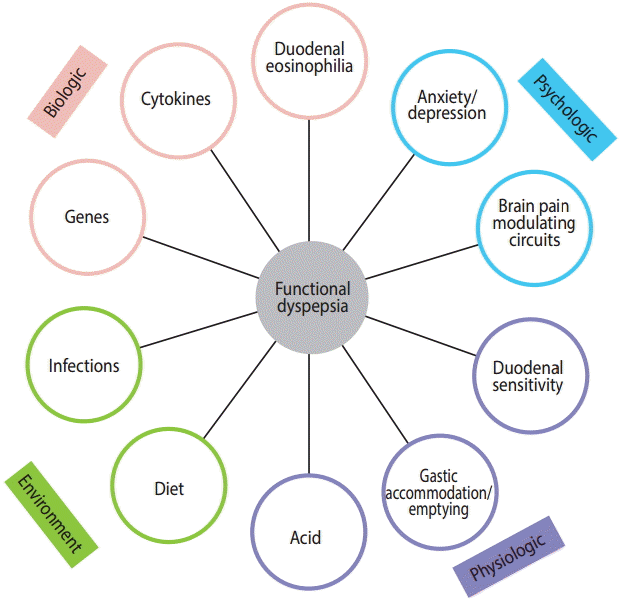
Over the past decade, there has been a dramatic change in how FD has been conceptualized, from a brain-gut motility disorder, in which the gut pathology is absent, to a gut driven disorder with observable gastrointestinal pathology [3]. Randomized controlled trials have confirmed that infection can cause FD, most notably Helicobacter pylori [4]. Post-infectious FD following bacterial gastroenteritis has also been recognized and may have a distinct pathophysiology, while the role of the microbiome has yet to be established but is likely to be important [5]. Emerging data suggest that up to 40% of patients with FD have a duodenal pathology, including an increase in eosinophils that may account for the symptoms [6,7]. Other data suggest that the systemic and psychological symptoms often experienced by patients with FD may, in some, be explained by cytokine release from an inflammatory focus [8]. In this review the epidemiology, pathophysiology and treatment of FD are summarized, with a particular focus on data published over the last decade.
DEFINITIONS
The Rome III criteria recommend defining FD based on the presence of one of more of four cardinal gastroduodenal symptoms, namely epigastric pain, epigastric burning, postprandial fullness, and early satiety (inability to finish a normal sized meal) [9]. The symptoms of FD present over the preceding 3 months and are chronic (of at least 6 months duration). Epigastric pain or burning are typical ulcer-like symptoms and fit closely with what was previously known as NUD. The Rome III criteria labels those with epigastric pain and/or burning as epigastric pain syndrome (EPS). Burning in the epigastrium should be distinguished from retrosternal burning (heartburn), although FD and gastroesophageal reflux disease (GERD) do overlap, as discussed below. Postprandial fullness and an inability to finish a normal sized meal (early satiety) are suggestive of gastroduodenal dysmotility; patients with one or both of these symptoms are classified by the Rome III criteria as postprandial distress syndrome (PDS).
While the Rome criteria do not consider bloating as a gastroduodenal symptom, the Asian Consensus Report on FD suggested including upper abdominal bloating, as it is a common feature in patients from the region [4]. However, many patients find it difficult, if not impossible, to locate their bloating in the upper abdomen, and bloating may arise from lower down in the gut [10]. Nausea is another common symptom that can occur in FD but also is common in other FGIDs and appears not to be a specific feature [11].
The Rome criteria for FD have been the subject of considerable criticism. The major objection is that they do not discriminate FD from peptic ulcers or other organic diseases known to cause dyspepsia, and this applies to all versions published to date [12]. The failure of the criteria to provide a positive diagnosis is far from surprising and likely reflects the limited repertoire of the body to respond to injury or pathophysiological disturbances. Nevertheless, there have been major advances over the last decade in the identification of PDS and EPS cases, as well as new findings on the pathogenesis of FD, as discussed below.
EPIDEMIOLOGY
Epidemiological studies have confirmed that EPS and PDS are distinct syndromes. A pivotal population-based study (the Kalixanda study, named after the towns of Kalix and Haparanda where the research was conducted) obtained a random sample from two northern Swedish towns where subjects were invited to complete validated questionnaires and undergo an unsedated EGD to exclude esophagitis, peptic ulcer, and cancer [13]. Of 1,000 representative community subjects from Kalix and Haparanda who underwent EGD, 4% had organic disease that explained their dyspepsia, while 16% had FD, of whom 5% had EPS and 12% PDS [14]. Other population-based studies from Europe and the United States have confirmed that PDS is more prevalent than EPS [15].
In Asia, epidemiological studies have also identified FD as a highly prevalent disorder [16,17]. In a Korean telephone survey of 5,000 subjects who completed the Rome III questionnaire, 7.7% had dyspepsia; postprandial distress was reported by 5.6% and EPS by 4.2%, while 27% had both PDS and EPS [18]. Other data from Asia and the West suggest that the overlap of PDS and EPS is much lower among those patients who consult their medical practitioners [3]. However, in outpatients presenting with FD, the overlap of PDS and EPS is more pronounced, and some patients are difficult to place into subgroups [19].
Risk factors for PDS and EPS remain to be fully characterized. In the Swedish population-based study, anxiety and nonsteroidal anti-inflammatory drugs (NSAIDs) were linked to PDS but not EPS [14]. Depression was not identified as an important risk factor for either subgroup. In an Italian population-based endoscopy study, smoking was linked to PDS but not EPS [15]. In contrast, in a large gastric cancer screening population from Taiwan, anxiety and NSAIDs were risk factors for both PDS and EPS, while H. pylori and depression were linked to PDS [20]. These data are in contrast with clinical trial data from Asia, which suggested that H. pylori responders were more likely to have EPS than PDS, but this finding requires confirmation [21]. If risk factors do differ between Eastern and Western populations, an explanation needs to be identified, as this may provide new hints regarding the pathogenesis. For example, dietary differences may impact the intestinal microbiome, and this may in turn modulate any inflammatory process that leads to FD. Little is known about the microbiome in FD [22].
Other intriguing associations remain to be confirmed. For example, an increased prevalence of joint hypermobility syndrome has been observed in FD, but rates were also surprisingly high in the controls [23]. A link to autoimmune disease is possible but needs to be verified [24].
The drivers leading people to seek consultation for FD are poorly defined. In a community study from Hong Kong, anxiety was identified as an independent risk factor for seeking health care [25], but other studies have not confirmed these observations [26,27]. The severity of the dyspepsia also drives people to seek care, although over 50% of affected individuals do not seek consultation [28] despite many believing they have a serious condition [29].
PATHOPHYSIOLOGY
The causes of FD remain to be established, but accumulating data suggest infections and possibly food may play an important role in a subset of individuals, as reviewed below (Fig. 1).
Meals induce symptoms in the majority of individuals
Food ingestion plays an important role in the genesis of FD symptoms, although the mechanisms remain to be elucidated. In a previous study, a standard solid meal was given to 218 tertiary care patients with FD, and symptom severity was measured every 15 minutes for 4 hours [30]. Even if patients failed to recognize a relationship between eating and their symptoms, nearly 80% had increased symptom intensity 15 minutes after the meal. Notably, the timing varied by symptom type, with fullness occurring much earlier than pain after the meal. Interestingly, those patients with EPS were also more likely to report a delayed peak in pain, suggesting they frequently have true meal-induced symptomatology, but it often goes unrecognized [30].
High-fat meals slow gastric emptying and can lead to dyspepsia, while moderate-to-fast eating and irregular meals are also associated with dyspepsia [31,32]. The role of disturbances in gut hormones in FD postprandially is uncertain, although increased cholecystokinin and ghrelin have both been implicated potentially [33,34].
Gastric perturbations
Traditionally, FD has been conceptualized as a motility disorder dominated by disturbances in gastric physiology. There are conclusive data suggesting that gastric emptying is delayed in a subset of people with FD, although the prevalence varies widely (from 10% to 40%, with a median of 25%) [3]. Occasionally, gastric emptying is fast rather than slow in FD, although most have normal emptying. Notably, symptoms correlate very poorly with slow gastric emptying in FD, suggesting the finding is likely an epiphenomenon [3,8,9].
Normally after a meal, the gastric fundus relaxes, allowing a pleasant feeling of satiation, and this is lost after vagotomy [3]. Relaxation of the gastric fundus is also impaired in a subset of people with FD, affecting up to 40% of cases (termed fundic disaccommodation), and this has been linked to early satiety in some but not all studies [3]. In a subgroup of FD, the stomach also exhibits hypersensitivity, as shown by distending a barostat balloon in the stomach; patients with FD feel the balloon at lower volumes and pressures than do controls, but the link to symptoms is tenuous [3,35]. Disturbed gastric physiology may alter food intake in FD; a lower body mass index has been observed in a subset of FD patients in some studies [36,37], and obesity is uncommon in this syndrome.
Duodenal perturbations
More recent research has focused on the duodenum, where disturbed motor and sensory functions have also been observed in patients with FD. Acid infusion into the duodenum can induce symptoms and motility changes in FD [38]. This was unexplained in adults, until observations of a subtle increase in eosinophils in the duodenum in FD were reported [39]. Talley et al. [39] hypothesized a priori that eosinophils are recruited to the duodenum secondary to duodenal acid or food allergen exposure in FD, and then degranulate, releasing toxic products, such as major basic protein, to signal local mucosal pain fibers. In the Kalixanda study, a quantitative increase in duodenal eosinophils was observed in cases with FD compared with the controls, with increased clusters of eosinophils in FD and degranulation adjacent to nerves in some cases. Counting of eosinophils is key, as eosinophilia is subtle, and the association would otherwise have been missed (as it has been in adults until recently) [39]. These initial observations have been confirmed in Western and Eastern populations [6,40-42], and research from Belgium has reported that increases in eosinophils are associated with increased mucosal permeability [43]. Further research has suggested that duodenal eosinophilia is linked to pain and early satiety, and the risk is increased in smokers [6]. A large-scale epidemiological investigation also associated FD with atopic conditions [44].
Perturbations in the duodenum may theoretically induce reflex responses that alter gastric physiology, possibly explaining the altered gastric emptying and other findings in FD [38]. Other research has shown that delayed gastric emptying in FD is linked to circulating small bowel homing T cells (CD4 + α4β7 + CCR9 + lymphocytes) as well as cytokine release (tumor necrosis factor α [TNF-α], interleukin 1β [IL-1β], and IL-10 secretion), supporting the view that small intestinal inflammation may be a primary driver of gastric dysfunction [8].
Infections
Post-infectious FD is a newly-recognized syndrome and can follow infection by several organisms, including, Salmonella, Escherichia coli, Campylobacter, giardiasis or norovirus, and possibly other upper intestinal infections as well [5]. Follow-up of cases after acute bacterial gastroenteritis has shown that those exposed to these infections have an approximately 2.5-fold increased risk of developing FD, and an increased risk of developing both FD and irritable bowel syndrome (IBS) [5]. Those who have a more severe infection or who smoke are at increased risk. Other data have shown that smokers have an increased risk of duodenal eosinophilia, which is also linked to FD [6].
It had been debated whether H. pylori is a cause of FD, but the evidence is now clear. While in the majority of cases of H. pylori, the associated histological gastritis appears to have no clinical effect, in an important minority, curing the infection leads to long-term symptom resolution even a year after completing eradication therapy [45]. A meta-analysis of randomized controlled trials concluded that H. pylori eradication is superior to the placebo (or short-term acid suppression), with a number needed to treat (NNT) of 17 [46]. The role of the microbiome in the pathogenesis of FD is otherwise largely unknown but may be altered by dietary changes or possibly inflammation [22].
Brain-gut disturbances
Psychological distress is associated with FD, with a strong link to anxiety [14,47]. The best evidence for the role of anxiety comes from longitudinal investigations of subjects free of FD symptoms. In the Kalixanda study, the development of FD was nearly 8-fold higher in those with baseline anxiety, suggesting a cause and effect relationship [48]. Early life factors are likely key, with the possibility of a vicious cycle developing in which mood disturbance exacerbates dyspepsia, which in turn increases the severity of the mood disorder [47].
In many cases, anxiety may precede the disorder (a brain-gut link), but in others, the gut symptoms may precede the anxiety (suggesting a gut-brain link) [48]. How might the gut alter brain-inducing anxiety? Symptoms may increase anxiety; alternatively, another potential mechanism is through cytokine release secondary to gut inflammation. For example, TNF-α was significantly increased and correlated with greater anxiety in IBS [49], while cytokine release has been linked to slow gastric emptying in FD [8].
Imaging studies have identified changes in the relevant brain regions where pain-modulating circuits exist, compared with the controls [50]. Assessing resting brain glucose metabolism by fluorodeoxyglucose positron emission tomography (PET) revealed higher metabolism levels in a number of brain regions considered to be part of the key pain modulatory circuits, including the anterior cingulate cortex, insula and thalamus. These changes correlated with symptom severity but not anxiety [51], although other studies have suggested that patients with anxiety or depression and FD demonstrate greater hypermetabolism in these circuits [52]. Structural changes in gray matter density have also been reported in PDS [53]. A limiting aspect of that research was the lack of controls with chronic non-gut pain to ascertain if the changes seen were disease-specific or reflected non-specific alterations with chronic pain. The central neurotransmitter systems involved in FD remain to be defined fully, but recent preliminary PET data have identified a higher presence of cerebral cannabinoid-1 receptors in FD, implicating sustained endocannabinoid system dysfunction, which may represent a new central therapeutic target [54].
Genetics
Limited data have implicated genetic factors in FD [55]. GNbeta3, which alters G-protein activation and multiple other pathways, was the first single nucleotide polymorphism (SNP) associated with FD [56], and a number of studies have confirmed a link, although the associations vary by population [55]. The nitric oxide synthase (NOS) gene (namely the T-allele of neuronal NOS) has also been linked to FD [57]. Other SNPs possibly linked to FD include those in TRPV1, which is related to acid sensitivity [58], and the tetrodotoxin-resistant sodium channel Na (V) 1.8, which is involved in nociception [58,59]. In post-infectious FD, Japanese investigators have linked a cyclooxygenase 1 polymorphism to EPS, but this remains to be confirmed [59,60]. Small sample numbers and inadequate validation remain issues for most of these associations [61].
A disease model for FD
It has been hypothesized that either a food allergen or infection in a genetically predisposed host can induce upper small intestinal permeability, leading to antigen presentation and immune activation, with a switch from a TH1 to a TH2 response [62-64]. This in turn leads to recruitment of eosinophils that degranulate, resulting in some, but not all, cases of FD developing focal tissue injury and producing cytokines and circulating homing T cells that induce gastric perturbations and satiety. Eosinophil degranulation may also directly trigger neural excitation, muscle contractions and pain. In a subset of FD cases, both mast cells and eosinophils may be recruited, and both cell types are a key link between innate and adaptive immunities. It is possible that in other cases, the eosinophils are protective and reduce injury, promoting healing and symptom resolution. This hypothetical pathway needs rigorous testing in animal and human models of the disease.
DIAGNOSIS
Diagnosis of FD remains one of exclusion. Data from Asia shows that 40% to 80% of patients presenting with dyspepsia were diagnosed with FD after a complete workup [65,66].
EGD can exclude esophagitis, peptic ulcer disease and esophagogastric cancers. The Asian consensus report recommends that all patients presenting with dyspepsia aged 40 years or over and in regions of high prevalence cancer should undergo EGD to exclude cancer [4]. Similarly, the consensus recommended EGD for all those presenting with alarming symptoms (e.g., weight loss, vomiting, dysphagia, bleeding, or a family history of cancer), although the positive predictive value of these alarming symptoms is low (i.e., most people presenting with one of these symptoms will not have cancer) [67].
Those with new onset dyspepsia who are young and have no alarming symptoms have the option to enter an empirical therapy trial, with a choice between testing and treating H. pylori or empiric acid suppression, depending on the local prevalence of H. pylori and gastric cancer, the health care treatment policy, and patient preference [4,68]. Unfortunately, endoscopy fails to provide reassurance to patients with FD, with no improvement in quality of life or psychological distress observed in a cohort both before and 1 month after endoscopy [69].
Differentiating GERD from FD is not straightforward, as esophageal and gastroduodenal symptoms often coexist. Many patients with GERD do not have esophagitis, but they may still have GERD (non-erosive reflux disease). In a Chinese study, pathologic acid reflux in the esophagus, as measured by ambulatory 24-hour pH monitoring, was detected in over 25% of patients with FD and was most prevalent in those with epigastric burning [70]. The symptoms of GERD occur more commonly in FD than expected by chance in the West and Asia, suggesting a shared pathophysiology [71]. Recently, a close link between transient lower esophageal sphincter relaxations (TLESRs), which occur in GERD, and gastric accommodation was observed, and it has been suggested that activation of mechanoreceptors is involved in triggering the TLESRs [72]. If gastric accommodation and GERD are linked through TLESRs, it would not be surprising that those with gastric accommodation dysfunction experience more heartburn, potentially explaining the close relationship between GERD and FD [72].
FD also overlaps with IBS more than expected by chance, including in Asia, suggesting that FD is part of a spectrum of FGIDs with similar pathophysiology, and thus attempting to identify each by symptoms alone is problematic [25]. A working hypothesis is that if eosinophils and mast cells are recruited into the small intestine, both FD and IBS may result, while if mast cells alone infiltrate, then IBS but not FD will occur [7].
In view of the association of FD with duodenal eosinophilia in Western countries in the absence of obvious parasites, it is interesting that parasitic infestation may present more commonly with dyspepsia in Asia, including giardiasis, ascariasis, fascioliasis, and opisthorchiasis [73]. However randomized controlled trials establishing a causal relationship are lacking. Hepatocellular carcinoma is prevalent in Asia because of hepatitis B infection and may manifest initially with dyspepsia [68].
Chronic dyspepsia may result from gastroduodenal disease, but pancreaticobiliary disease should also be considered in the correct clinical setting [74]. Biliary pain can be epigastric but is usually more severe, episodic, and unpredictable and may radiate to the back or shoulder. Pancreatic pain may also be confused with EPS. Celiac disease may be confused with FD in some cases, although a true association remains to be confirmed [75]. Side effects from drugs (e.g., from NSAID ingestion) rarely causes chronic dyspepsia that is confused with FD [76]. Duodenal eosinophilia may be a useful diagnostic marker in the correct clinical setting, but its value in clinical practice remains to be confirmed [40].
TREATMENT
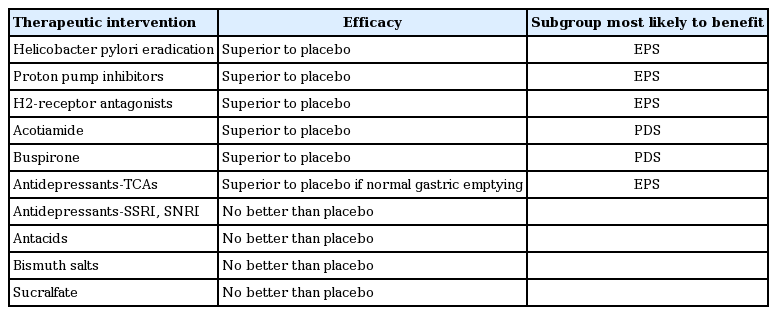
Once a firm diagnosis of FD has been established, it is clinically useful to subdivide FD into EPS or PDS when planning treatment (Table 1) [77]. All patients with FD should be reassured that FD is not associated with any increase in mortality [78]. Dietary change may help in some cases, including smaller regular meals and reduced fat intake (fat can slow gastric emptying) [32,33]. Reducing anxiety and addressing other psychological issues is often helpful, and formal psychological interventions should be considered in cases with resistant symptoms [48,79,80].
Testing for and treating H. pylori
Although it has been controversial, it is reasonable to test for H. pylori in all FD cases and treat the infected cases [68]. Eradication therapy only helps a minority of cases (NNT, 17) [81], but as the benefits are long-term, the risk-to-benefit ratio is arguably favorable [82,83]. Further, eradication of the infection reduces the risk of future peptic ulcer disease (and missed peptic ulcers may explain a minority of cases with suspected FD, as the ulcer may have healed by the time endoscopy is undertaken), and eradication may reduce the risk of gastric cancer if atrophic gastritis has not supervened [68]. There is evidence that H. pylori may be more strongly linked to EPS, but this is controversial [84]. Admittedly, no eradication therapy trials have been conducted in H. pylori-negative FD cases to confirm that the benefit is not related to any effects of other gut bacteria.
If an infection is found, offering eradication therapy and re-testing at least 1 month after therapy cessation to confirm that a cure is reasonable. Current first line eradication therapy fails to cure the infection in more than 30% of cases [85]. In those who are cured and remain so 12 months post-therapy, this arguably suggests they never had FD but rather suffered from H. pylori-induced organic dyspepsia [68], although this is more an argument of semantics.
Epigastric pain syndrome: first line
Acid suppression reduces FD symptoms compared with a placebo, and meta-analysis data suggest that EPS is most likely to respond [81]. Proton pump inhibitor (PPI) therapy and H2 receptor antagonist therapy are both superior to a placebo, with H2 blockers having a larger benefit, but the data are more heterogeneous [81].
Notably, while it is used most widely and recommended most often, only a minority of individuals respond to PPIs, and their long-term use can have adverse side effects [81]. Most studies are based on Western populations, but a randomized placebo-controlled trial from Hong Kong in young subjects with uninvestigated epigastric pain who were H. pylori-negative failed to demonstrate a benefit of PPI therapy [86], and similar results were reported overall for FD [87]. A Japanese trial showed that 15 mg lansoprazole was superior to a placebo for FD, but only EPS responded to the drug [88]. Another trial from Japan failed to demonstrate any differences by subgroup, although FD responded to 20 mg rebaprazole, but not to the other doses tested [89].
If a PPI fails after an adequate full-dose therapy trial, it is reasonable to try a H2 receptor antagonist. An alternative is to try a prokinetic, especially if the symptoms of EPS and PDS overlap [90].
Postprandial distress syndrome: first line
A prokinetic is usually offered as a first line treatment, but options vary by country and remain restrictive. Very limited data support the use of domperidone, a dopamine antagonist, and the same applies to metoclopramide [77,91]. Cisapride, a serotonin type 4 (5HT4) agonist and serotonin type 3 (5HT3) antagonist, has been withdrawn in most countries because of QT prolongation, but it was superior to a placebo in cases with FD [81]. Tegaserod, a 5HT4 agonist, was marginally better than a placebo [90], while mosapride is probably not efficacious [92], and itopride is of uncertain benefit over a placebo [93].
Acotiamide is approved for FD in Japan. The drug relaxes the gastric fundus and accelerates gastric emptying in humans [94]. A modest but significant benefit over a placebo was observed in FD, with 52% of those on acotiamide for over 4 weeks responding, compared with 35% on the placebo, based on a global assessment of the overall treatment effect [95]. Complete loss of meal-related symptoms occurred in 15% of those on acotiamide versus 9% on a placebo, which was significant statistically but arguable in terms of clinical efficacy [95]. Other drugs also relax the gastric fundus and may have a value in treating FD, including the anti-anxiety drug and serotonin type 1a agonist buspirone and tandospirone, and the anti-migraine drug and serotonin type 1 agonist, sumatriptan [96,97], but data remain limited.
Centrally acting therapy
If first line therapy fails, a centrally acting drug may be considered. In the Functional Dyspepsia Treatment Trial conducted in North America, a low dose tricyclic antidepressant (amitryptiline 50 mg) was compared with a selective serotonin reuptake inhibitor (SSRI) in antidepressant dosing (escitalopram 10 mg) and a placebo over 3 months with a 6-month follow-up post-therapy [98]. The SSRI performed no better than the placebo, which was consistent with other clinical data on a selective serotonin norepinephrine reuptake inhibitor (SNRI), venlafaxine [99]. On the other hand, the tricyclic antidepressant appeared to provide a modest benefit for FD, especially in those with pain [98]. Patients with ulcer-like FD, likely equivalent to EPS, who were given amitriptyline were over 3-fold more likely to report adequate relief than were those who received the placebo, although the patient diaries showed that meal-related symptoms also benefited compared with the placebo. Interestingly, tricyclics can theoretically slow gastric emptying, and those with delayed gastric emptying were less likely to report adequate relief on amitriptyline compared with FD patients with normal emptying. Notably, antidepressant therapy did not change psychological distress measures, and did not alter gastric emptying rates.
Another promising approach is the use of the teracyclic antidepressant mirtazepine. Although mirtazepine does not alter gastric function [100], results from a preliminary randomized controlled trial of FD suggest benefits over a placebo [101]. Levosulpiride is an atypical anti-psychotic drug that is a benzamide derivate and dopamine [2] antagonist; in cases with FD, it may be as efficacious as cisapride, but more data are needed, including large placebo controlled trials [102].
Psychological therapies are of uncertain benefit in FD, although positive trial data have been reported [103]. However, combining medical therapies with psychological therapy may provide better outcomes, although large-scale trials are needed to confirm these observations [79,104].
Anti-inflammatory therapy
Little data exist on the use of anti-eosinophil therapies in FD. Anecdotally, anti-histamine therapy has helped some patients with FD. In children, eosinophil stabilization using montelukast did not reduce eosinophil numbers but did reduce symptoms [105].
Alternative therapy
Herbal products have been tested for their effectiveness in FD, with variable and often negative results [106]. The combination herbal product iberogast (STW-5, Medical Futures Inc., Richmond Hill, ON, Canada) relaxes the gastric fundus [106] and was superior to a placebo in a randomized controlled trial, although the quality of the randomized trial data were low [107,108].
Rikkunshito is a Japanese herbal medicine that increases acyl ghrelin levels and may improve FD symptoms, although this appears to be restricted to H. pylori-infected individuals and requires confirmation [109]. Overall, convincing data supporting the use of any herbal therapies for treatment of FD are lacking. An intriguing approach is to pass an electric current through the abdomen, which in one small randomized controlled trial, appeared to reduce the symptoms of pain and meal-related complaints, but the findings need to be confirmed [110].
CONCLUSIONS
FD is a common disorder affecting every one in 10 of the general population. Management relies on an accurate diagnosis, including ruling out less common causes of similar symptoms, followed by reassurance and approaches to reduce stress and modify any dietary triggers. Once FD is confirmed, H. pylori can be identified reasonably and infected cases treated, although only a minority will have true H. pylori-induced dyspepsia and respond to treatment. In those with EPS, acid suppression therapy is a first line therapy, but a H2 blocker is worth a trial even if PPI fails. In PDS, a prokinetic is preferred, but switching between these drug classes should be considered if the therapy fails. A second line therapy includes administration of a tricyclic antidepressant in low doses, or mirtazapine, but not an SSRI or SNRI.
Notes
NJT has research support from GI Therapies, Janssen, Prometheus, Pfizer and Salix, and has consulted for Adelphi Values, Allergens, GI Therapies and Yuhan.