Comparison of intraductal ultrasonography-directed and cholangiography-directed endoscopic retrograde biliary drainage in patients with a biliary obstruction
Article information
Abstract
Background/Aims:
Endoscopic retrograde biliary drainage (ERBD) has become a standard procedure in patients with a biliary obstruction. Intraductal ultrasonography (IDUS) has emerged as a new tool for managing extrahepatic biliary diseases. IDUS-directed ERBD can be performed without conventional cholangiography (CC). The goal of this study was to assess the effectiveness and safety of IDUS-directed ERBD compared to CC-directed ERBD in patients with an extrahepatic biliary obstruction.
Methods:
A total of 210 patients who had undergone IDUS-directed ERBD (IDUS-ERBD, n = 105) and CC-directed ERBD (CC-ERBD, n = 105) between October 2013 and April 2014 were analyzed retrospectively. The primary outcome measure was the procedural success rate. Secondary outcome measures included clinical outcomes, total procedure time, radiation exposure time, and overall complication rates.
Results:
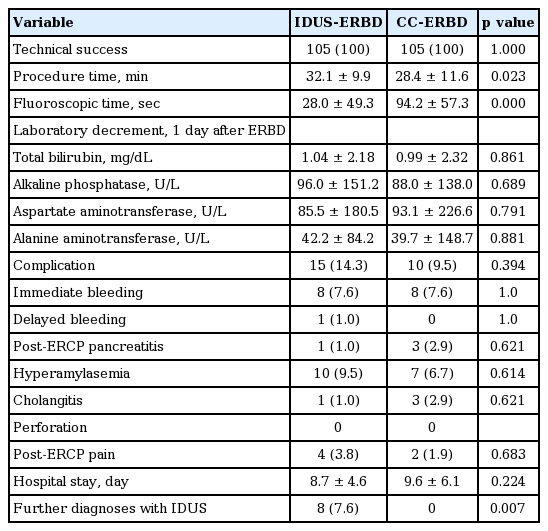
The total technical success rate of ERBD was 100% (105/105) in the IDUS-ERBD and CC-ERBD groups. Mean procedure time was slightly prolonged in the IDUS-ERBD group than that in the CC-ERBD group (32.1 ± 9.9 minutes vs. 28.4 ± 11.6 minutes, p = 0.023). Mean radiation exposure time was one-third less in the IDUS-ERBD group than that in the CC-ERBD group (28.0 ± 49.3 seconds vs. 94.2 ± 57.3 seconds, p < 0.001). No significant differences in complication rates were detected between the groups.
Conclusions:
IDUS-ERBD was equally effective and safe as CC-ERBD in patients with an extrahepatic biliary obstruction. Although IDUS-ERBD increased total procedure time, it significantly decreased radiation exposure.
INTRODUCTION
Endoscopic retrograde cholangiopancreatography (ERCP) is a standard technique for managing of biliopancreatic diseases [1,2]. Endoscopic retrograde biliary drainage (ERBD) is usually performed under conventional cholangiography (CC) for relief of a biliary obstruction and to ameliorate clinical symptoms. However, CC carries hazards related to radiation exposure and contrast media [3]. CC cannot effectively differentiate small stones or sludge from air bubbles [4]. In addition, CC cannot accurately define the nature of a stricture or the longitudinal spread of cholangiocarcinoma [5].
With the development of optical technology, high resolution intraductal ultrasonography (IDUS) provides more detailed imaged of the biliopancreatic tree and adjacent structures. The flexibility and small diameter of IDUS along with detailed image quality is ideal for evaluating biliopancreatic diseases, such as suspected intraluminal filling defects, indeterminate biliary strictures, and periampullary neoplasms [6]. The sensitivity of IDUS in patients with choledocholithiasis is superior to those of ERCP and abdominal ultrasonography. Cannulation with the IDUS probe into the biliary tree can be performed in most patients without prior endoscopic sphincterotomy (EST). IDUS-directed ERBD can be performed without CC [7,8].
The purpose of this study was to assess the effectiveness and safety of IDUS-directed ERBD (IDUS-ERBD) compared to CC-directed ERBD (CC-ERBD) in patients with an extrahepatic biliary obstruction.
METHODS
Patients
A total of 210 patients who had undergone IDUS-ERBD (n = 105) and CC-ERBD (n = 105) to prevent extrahepatic biliary obstruction between October 2013 and April 2014 at Chonnam National University Hospital were analyzed retrospectively. Biliary obstruction was diagnosed based on clinical symptoms, laboratory tests, and imaging (abdominal ultrasonography, abdominal computed tomography [CT], magnetic resonance cholangiopancreatography, and biliary endoscopic ultrasonography). ERBD was performed in patients with various clinical conditions, including choledocholithiasis, obstructive cholangitis, and a malignant biliary obstruction. During the study period, a total of 452 patients underwent ERCP for the treatment of choledocholithiasis and cholangitis. Among them, 294 patients (65%) were treated by EST and/or endoscopic papillary balloon dilatation (EPBD) alone, and the other 158 patients (35%) were treated by EST and/or EPBD plus ERBD due to difficult common bile duct (CBD) stones until second session ERCP. Written informed consent was obtained from all patients. This study was approved by the Institutional Review Board of Chonnam National University Hospital, Gwangju, Korea (IRB No. CNUH-2014-121).
Methods
After general supportive care for biliary obstruction, either IDUS-ERBD or CC-ERBD was performed by two experienced endoscopists. All ERCP procedures were performed using a standard side-viewing duodenoscope (TJF-160F, Olympus, Tokyo, Japan) in an endoscopy room. Selective bile duct cannulation was performed with a 0.035-inch-diameter guidewire (Jagwire, Boston Scientific, Natick, MA, USA). After cannulation with a guidewire, the “bile aspiration” technique was used to indicate bile duct cannulation. A 2.0-mm-diameter IDUS probe with a frequency of 20-MHz (UM-G20-29R, Olympus) was advanced over a guidewire into the bile duct during IDUS-ERBD. A plastic stent (Percuflex DUODENAL BEND Biliary Stent, Boston Scientific) for ERBD was inserted over the guidewire to the proper position after withdrawal of the IDUS probe. The length of a plastic stent was determined by the insertion length of the IDUS prove from the papilla of Vater to the lesions. A conventional cholangiogram was obtained after selective biliary cannulation during CC-ERBD. After confirming biliary lesions, an indwelling plastic ERBD stent was introduced over the guidewire. A linear or bi-pigtailed biliary stent (caliber, 5 to 10 Fr; size, 7 to 12 cm) was released appropriately according to the lesion site. The location of ERBD was confirmed with plain radiography.
After the ERCP procedures, laboratory findings, including serum amylase, total bilirubin, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, complete blood cell count, abdominal radiographs, and abdominal CT were checked to monitor for complications, such as acute pancreatitis, acute cholangitis, bleeding, and perforations. Procedural-related pancreatitis was defined as abdominal pain with at least a three-fold elevation in serum amylase > 24 hours after the procedure [9].
Baseline demographics and clinical characteristics were recorded before the procedures. The primary outcome measure was procedure success rate. Secondary outcome measures included clinical outcomes, total procedure time, radiation exposure time, and overall complication rates.
Statistical analysis
Data are expressed as mean ± standard deviation and percentages. The independent sample t test was used to compare means. The statistical analysis was performed using SPSS version 20.0 (IBM Co., Armonk, NY, USA). Binary variables were compared with the chi-square test. A p < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
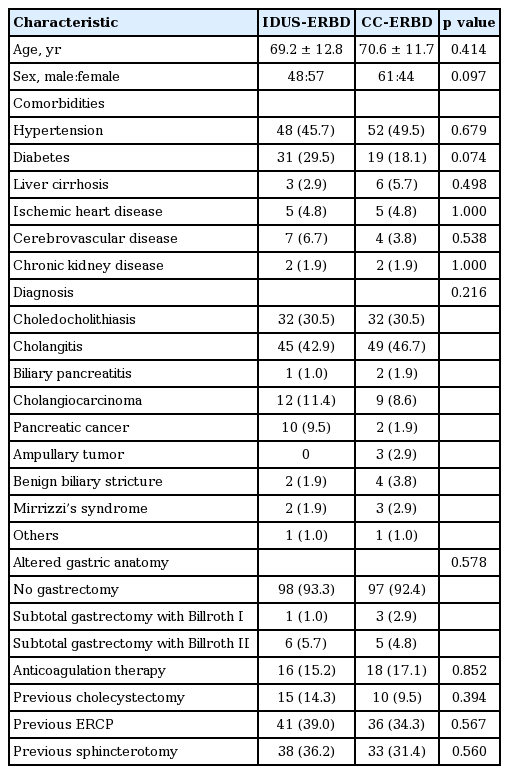
The baseline characteristics of the patients undergoing ERBD are categorized in Table 1. No significant differences were detected in the demographic data, clinical diagnoses, previous anticoagulant therapies, pervious cholecystectomy histories, or previous ERCP and EST.
Endoscopic findings on ERCP
The endoscopic findings on ERCP are shown in Table 2. Altered gastric anatomy due to previous gastrectomy was not different between the two groups. The presence of a periampullary diverticulum and the anatomical shape of the papilla were not different between the groups. Bile duct diameter was not different between the groups.
Clinical outcomes and complications
The total technical success rate of ERBD was 100% (105/105) for both the IDUS-ERBD and CC-ERBD groups. The second session ERCP was not needed due to malposition of the plastic stents in all patients. The mean procedure time was prolonged slightly in the IDUS-ERBD group compared to that in the CC-ERBD group (32.1 ± 9.9 minutes vs. 28.4 ± 11.6 minutes, p = 0.023). Mean radiation exposure time was one-third less in the IDUS-ERBD group than that in the CC-ERBD group (28.0 ± 49.3 seconds vs. 94.2 ± 57.3 seconds, p = 0.000). No significant difference was detected in the percentage of difficult cannulation procedures between the two groups.
An EST was needed in 25.2% of IDUS-ERBD cases to insert the IDUS probe. Concomitant procedures, such as brush cytology or biopsy, were more frequently performed in the IDUS-ERBD group than those in the CC-ERBD group (21.9% vs. 7.6%, p = 0.006).
Rates of complications, such as immediate or delayed bleeding, post-ERCP pancreatitis, hyperamylasemia, cholangitis, post-ERCP pain, and perforation, were not different between the two groups. However, the IDUS-directed procedure provided further diagnoses in eight patients, including sludge, Mirrizzi’s syndrome, papillary stenosis, distal CBD cancer, pancreatic head cancer, and extrinsic compression (7.6% vs. 0%, p = 0.007). The clinical outcomes and complications are summarized in Table 3.
DISCUSSION
Our results demonstrate the usefulness and safety of the IDUS-ERBD technique for managing a biliary obstruction. The technical success rate of IDUS-ERBD was 100%, which was comparable to that for standard CC-ERBD. In addition, IDUS-ERBD was successfully carried out in all cases without significant complications, such as pancreatitis, cholangitis, or bleeding. The clinical outcomes following ERBD were not different between the two groups. Total procedure time was prolonged slightly using the IDUS-ERBD approach, but the difference was < 4 minutes. Although total procedure time was prolonged mostly by the IDUS procedure, the prolongation of total procedure time in the IDUS-ERBD group may be attributed to the additional concomitant procedures, such as brush cytology or biopsies. Mean radiation exposure time was one-third less in the IDUS-ERBD group than that in the CC-ERBD group.
ERCP with fluoroscopy guidance is a well-established technique for biliary drainage in patients with a bile duct obstruction. The IDUS-ERBD approach has some advantages compared with the CC-directed approach. First, it protects against radiation hazards by minimizing radiation exposure time in patients and the procedure team members, including endoscopists, radiologists, and nursing assistants [10]. Previous reports have demonstrated a linear relationship between radiation dose and fluoroscopy time [11]. ERBD with stent insertion may prolong radiation exposure time to confirm proper placement. Stent insertion is the only independent predictor significantly associated with prolonged fluoroscopy [12]. In the present study, plastic stents were deployed for acute obstructive cholangitis without CC using IDUS guidance in the IDUS-ERBD group. Therefore, the IDUS-ERBD approach significantly decreased radiation exposure time to less than one-third that of the CC-ERBD approach (28.4 seconds vs. 94.2 seconds, p < 0001). Second, the IDUS-ERBD approach can be used as a salvage drainage procedure in patients with severe allergy to iodine because IDUS-ERBD does not use contrast media for ultrasonic cholangiography [13]. The reported incidence of adverse reactions to intravenous iodine-contrast media is 12.6% for mild reactions and 0.22% for severe reactions [14]. It has been demonstrated from experience with iodine contrast used during ERCP that, after this procedure, iodine can be detected in the bloodstream [15]. There have been documented episodes of adverse reactions to iodine-containing contrast media following ERCP [16]. In addition, it may reduce post-ERCP pancreatitis and cholangitis by not using contrast media [17,18]. One of the best ways to decrease the incidence of post-ERCP pancreatitis and cholangitis is to avoid injecting contrast media for cholangiography. However, we did not find differences in the rates of cholangitis, pancreatitis, and hyperamylasemia after the ERBD procedure between the groups. Third, IDUS can provide further information about the biliary tree or ampulla, even for subtle changes, such as small sandlike stones or sludge, small polypoid masses, and details of biliary stricture [6]. Therefore, there was significantly more brush cytology or biopsy in the IDUS-ERBD group compared with CC-ERBD group. Our results show that IDUS provided further diagnoses in eight patients including CBD sludge, extrinsic compression, and malignant stricture in the biliary tree. IDUS can effectively detect small stones < 5 mm compared to those detected by CC. In addition, IDUS can distinguish air bubbles from sludge on ultrasonic cholangiography [4]. IDUS can distinguish a benign from a malignant biliary stricture based on several ultrasonographic criteria, such as disrupted normal bile duct layers, heterogeneous internal echo texture, irregular outer borders, and hypoechoic mass [19-21]. Therefore, IDUS has a potential role for deciding on surgical intervention [22]. Finally, the IDUS-directed technique can be used without fluoroscopic facilities. Therefore, it can be performed at bed side for severely ill patients who cannot move from the intensive care unit [23].
The present study had some limitations. First, the study was a non-randomized retrospective single center study. Second, IDUS-ERBD without CC was performed by a single expert. Third, IDUS-ERBD also has some technical limitations. For example, some patients with tight stricture in the papilla or distal CBD and a large juxtapapillary diverticulum can limit IDUS cannulation [24,25]. An additional EST for inserting the IDUS probe was needed in 25.2% of cases. This result was similar to a previous report that introduced a small-diameter IDUS probe in 75% to 80% of patients without EST [5]. At last, regarding the cost of IDUS-ERBD, the added cost is $208 US dollar compared with that of the CC-ERBD.
KEY MESSAGE
1. Endoscopic retrograde biliary drainage (ERBD) under guidance of intraductal ultrasonography (IDUS) or conventional cholangiography is an equally effective and safe method in patients with a biliary obstruction.
2. The technical success rate and clinical and laboratory outcomes were comparable between the approaches.
3. Although IDUS-ERBD increased total procedure time, it significantly decreased radiation exposure time.
4. The IDUS-ERBD technique seems to be a more suitable option for detecting subtle changes in the biliary tree or papilla and to detect the presence of an indeterminate biliary stricture.
Notes
No potential conflict of interest relevant to this article was reported.