BCR-ABL1 transcripts (MR4.5) at post-transplant 3 months as an early predictor for long-term outcomes in chronic myeloid leukemia
Article information
Abstract
Background/Aims
The aim of this study was to identify the role of BCR-ABL1 transcript level as a predictor for post-transplant relapse and outcome in patients who underwent allogeneic stem cell transplantation (SCT) for chronic phase (CP) chronic myeloid leukemia (CML).
Methods
Of 101 patients receiving allograft in CML CP, 85 had available quantitative reverse transcriptase polymerase chain reaction data at post-transplant 3 months. These patients were divided into two groups according to molecular response (MR4.5), defined as a BCR-ABL1 transcript level ≤ 0.0032% on the international scale, at 3 months based on receiver operating characteristic curve analysis of relapse.
Results
The 4-year overall survival and event-free survival (EFS) were 80.6% and 57.3%, respectively, and the cumulative incidence of relapse at 4 years was 29.6% after a median follow-up of 126.4 months. We performed multivariate analyses including potential variables to evaluate the early predictive role of MR4.5 at 3 months and found that MR4.5 at 3 months was associated with a higher EFS (p = 0.028) and showed a trend for a lower relapse rate (p = 0.089).
Conclusions
our results imply that frequent molecular monitoring and immune suppressive therapy modulation are required for patients without reduction of BCR-ABL1 transcripts to this level after SCT.
INTRODUCTION
Since imatinib mesylate (IM), the first BCR-ABL1 tyrosine kinase inhibitor (TKI), became a first-line therapy for chronic phase (CP) chronic myeloid leukemia (CML), more potent second generation (2G) TKIs have been introduced into routine practice. However, allogeneic stem cell transplantation (SCT) remains an important treatment for patients with advanced-phase CML at diagnosis, those with a T315I mutation in BCR-ABL1, or those who fail to respond durably to TKIs [1]. Nonetheless, relapse after allogeneic SCT is observed in 20% to 40% of patients [2-4]. Previous studies showed the role of TKIs as an option for salvage therapy in patients with post-transplant relapse [5,6] and emphasized the importance of post-transplant monitoring by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR).
Several studies have suggested that early detection of BCR-ABL1 transcripts by qRT-PCR is associated with an increased risk of relapse [7,8]. Asnafi et al. [8] evaluated the predictive role of day 100 BCR-ABL1 quantification using qRT-PCR, whereas other studies emphasized serial measurement of BCR-ABL1 transcripts [9,10].
However, the sensitivity of PCR technology has recently increased and more stringent standardization of PCR assays is now available [11]. In an IM discontinuation study of post-transplant relapse that included transplant patients who sustained undetectable molecular residual disease (UMRD) for more than 2 years from IM therapy, we found an association between a previous allograft and sustained molecular response. This implies the importance of immunologic effects, such as donor-derived cytotoxic T-cells, in CML patients receiving allogeneic SCT [12]. Therefore, it is necessary to identify early predictors for post-transplant relapse.
The purpose of this study was to identify a uniform BCR-ABL1 transcript cutoff on the international scale (IS) that predicts post-transplant relapse and outcomes in patients who undergo allogeneic SCT for CP CML.
METHODS
Patient selection
A total of 110 consecutive patients with CML underwent allogeneic SCT at Seoul St. Mary’s Hospital between May 2001 and December 2013. Because our aim was to investigate an early predictor for post-transplant relapse in CP CML, nine patients with advanced disease: six in accelerated phase and three in blast phase (BC) at the time of transplant were excluded and 101 CP patients were finally evaluated. All of the patients received grafts from either a human leukocyte antigen (HLA) identical sibling or an unrelated donor, and HLA matching for unrelated SCT was based on molecular typing for HLA-A, -B, -C, and DRB1.
The transplantation procedures were performed as described below. Thirty-seven patients were given a reduced-intensity conditioning regimen consisting of either fludarabine (150 mg/m2) with intravenous busulfan (6.4 mg/kg) or fludarabine (125 mg/m2) with cyclophosphamide (120 mg/kg). Sixty-four patients were given a standard conditioning regimen consisting of either fractionated total body irradiation (total body irradiation 1,200 cGy) and cyclophosphamide (120 mg/kg) or intravenous busulfan (12.8 mg/kg) and cyclophosphamide (120 mg/kg). Prophylaxis for graft-versus-host disease (GVHD) was administered using a combination of short-course methotrexate with either cyclosporine (for related transplants) or tacrolimus (for unrelated transplants). Granulocyte colony stimulating factor (5 μg/kg) was subcutaneously administered daily to all patients from day +7 after transplantation until the absolute neutrophil count (ANC) was > 3.0 × 109/L. This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital.
Molecular monitoring
After allogeneic SCT, molecular testing by qRT-PCR was performed at 1, 3, 6, 9, and 12 months and subsequently at 6-month intervals if the patient did not experience relapse. Results were expressed as the ratio of BCR-ABL1 copy number to ABL1 copy number on the IS. For patients who lacked available records for BCR-ABL1 transcript at 1 and 3 months post-transplant, cryopreserved samples (cells or mRNA) were used for qRT-PCR testing. The quality of RNA was assessed using Experion automated electrophoresis (Applied Bio-Rad, Hercules, CA, USA), and only qRT-PCR results with more than 50,000 ABL1 transcripts were analyzed. Major molecular response was defined as a BCR-ABL1 transcript level of ≤ 0.1% on the IS. Molecular response (MR4.5) was defined as a reduction in the BCR-ABL1 transcript level to ≤ 0.0032%.
Definitions
Disease phase and response were defined according to recent criteria [1]. To examine post-transplantation outcomes, neutrophil engraftment was defined as the first of 3 consecutive days with an ANC > 0.5 × 109/L, and platelet engraftment was defined as the first of 5 consecutive days with a platelet count > 20 × 109/L without transfusion support. Failure to engraft by day 28 was considered a primary engraftment failure, and secondary engraftment failure was defined as initial engraftment with documented donor-derived hematopoiesis followed by the loss of graft function without disease recurrence. Acute GVHD (aGVHD) and chronic GVHD (cGVHD) were graded according to clinical consensus criteria [13,14]. Overall survival (OS) was calculated from the day of transplantation, with patients alive at the time of last follow-up being administratively censored, and event-free survival (EFS) was counted from day 0 to any type of relapse or death while in remission. Relapse was defined by ratio of BCR-ABL1 to ABL1 > 0.1% on the IS for two consecutive analyses and therapeutic intervention was made. Transplant-related mortality (TRM) was defined as death due to any cause other than relapse.
Statistical analysis
The aim of this study was to identify a predictive marker for post-transplant relapse and outcomes based on BCR-ABL1 transcript levels on the IS at one early time point in patients that underwent allogeneic SCT for CP CML. Probabilities of OS and EFS were calculated by the Kaplan-Meier method and compared by the log-rank statistic, whereas those for TRM and relapse were plotted according to cumulative incidence estimates and compared with the Gray test. The prognostic significance of presenting and transplantation covariates with respect to OS and EFS were determined using the Cox proportional hazard regression model, including variables with a p ≤ 0.1 from univariate analyses. Factors were considered to be statistically significant if they had an associated p < 0.05 as determined by the likelihood ratio test. The prognostic significance of covariates affecting the competing events of TRM and relapse were determined using the proportional hazard model for the sub-distribution of competing risks. The incidence of aGVHD and cGVHD was calculated using cumulative incidence estimates. Statistical studies were performed with the SPSS version 13.0 (SPSS Inc., Chicago, IL, USA) and the cumulative incidence analyses were carried out with R (freely distributed on the web, http://cran.r-project.org/).
RESULTS
Patient demographics and characteristics
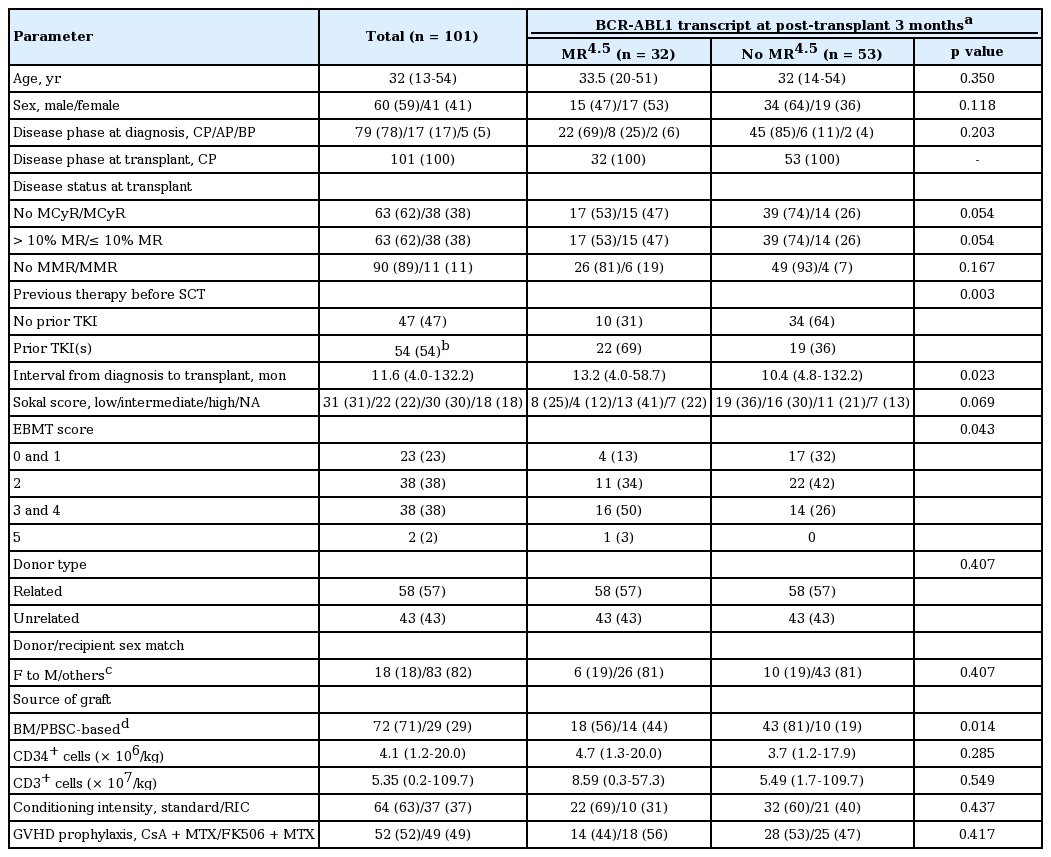
The study included a total of 101 patients (60 men and 41 women) with a median age of 32 years (range, 13 to 54). Of the 101 patients, 47 were TKI-naïve at the time of transplantation, in most case during the period before the national health insurance program covered IM, and 51 received IM as their frontline therapy. The remaining three received frontline treatment with 2G-TKIs: one with dasatinib (DAS), one with nilotinib, and one with bosutinib. Upon the failure of front-line TKI therapy, 17 patients were given other TKIs as second-line therapy, of whom eight were administered a third-line TKI. All patients received grafts from either matched siblings (n = 58) or unrelated donors (n = 43) (Table 1).
Prediction of relapse using BCR-ABL1 transcript levels at 3 months after SCT
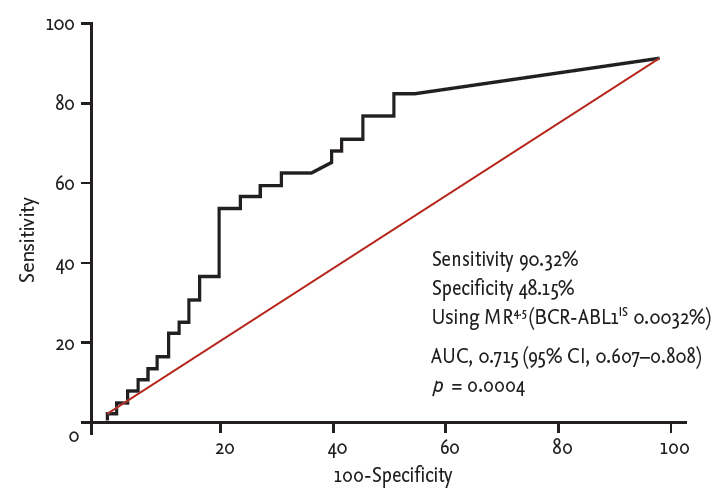
To evaluate the predictive role of the BCR-ABL1 transcript levels at 3 months after SCT, 85 patients who had available qRT-PCR data at 3 months after SCT were analyzed. We performed a receiver operating characteristic (ROC) curve analysis based on results of the association between BCR-ABL1 transcript levels at 3 months and relapse (Fig. 1). With consideration of the predictive marker as a test for post-transplant relapse in higher risk patients, the MR4.5 cutoff was chosen in favor of sensitivity over specificity. At 3 months after SCT, 32 patients achieved MR4.5 (MR4.5 group) and 53 patients did not achieve MR4.5 (no MR4.5 group). In comparison of clinical characteristics, the two groups were comparable for sex distribution, age, disease phase at diagnosis and transplant, and Sokal score, but in the MR4.5 group, prior TKI therapy was more frequent and the median interval from diagnosis to transplant was longer. In terms of transplant characteristics, there were no differences in conditioning intensity, donor type, and GVHD prophylaxis between the two groups. However, patients in the MR4.5 group showed a higher European Group for Blood and Marrow Transplantation (EBMT) score and more frequent use of peripheral blood stem cells (PBSCs) as a source (Table 1).
Engraftment and GVHD
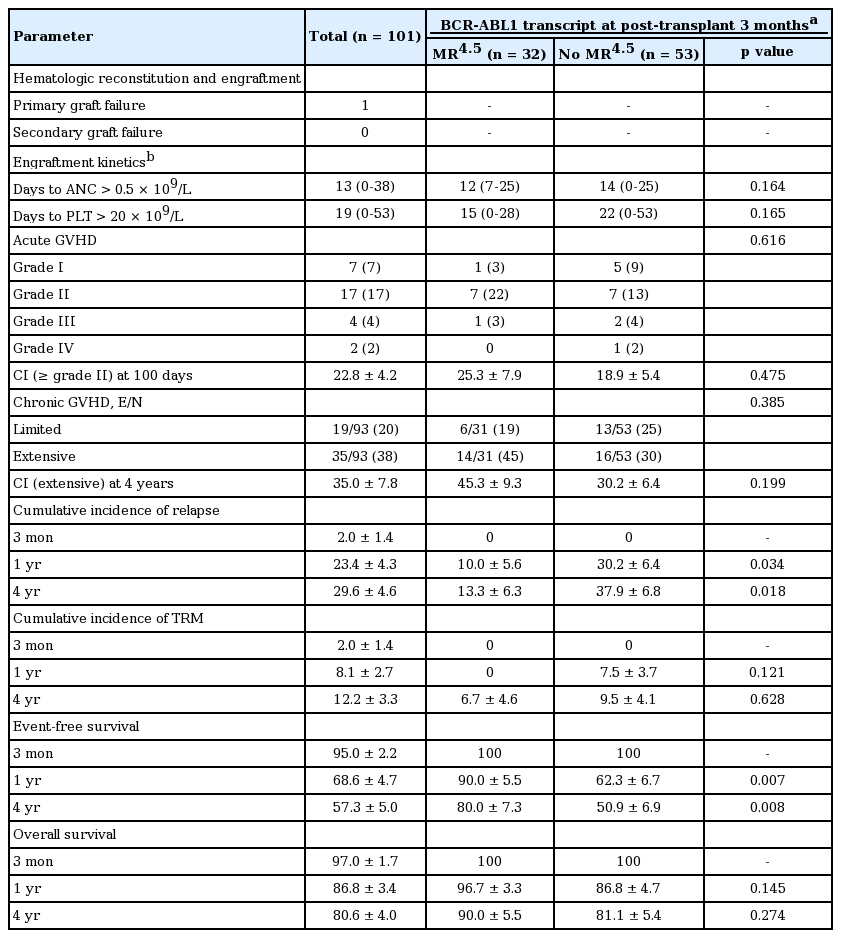
Transplant outcomes are shown in Table 2. All patients except for one achieved primary engraftment. The median times to neutrophil and platelet engraftment were 13 and 19 days after SCT, respectively. The cumulative incidence of clinically significant aGVHD (≥ grade II) at 100 days was 22.8%, and of the 93 patients who were evaluated, chronic extensive GVHD developed in 35 patients (38.0%). The 4-year cumulative incidence of chronic extensive GVHD was 35.0%. There were no differences in the occurrence of aGVHD and cGVHD between the two groups (MR4.5 group vs. no MR4.5); cumulative incidence of aGVHD (≥ grade II) at 100 days was 25.3% and 18.9%, respectively (p = 0.475) and cumulative incidence of cGVHD at 4 years was 45.3% and 30.2% (p = 0.199).
Prediction of outcomes by MR4.5 at post-transplant 3 months
With a median follow-up of 126.4 months (range, 3.1 to 154.8) for survivors, the 4-year OS and EFS was 80.6 % and 57.3%, respectively. Of the 101 patients, 12 died of transplant-related toxicities after transplantation, including aGVHD (n = 3), cGVHD (n = 6), infections (n = 2), and veno-occlusive disease (n = 1), and nine patients died after post-SCT relapse. Of the 101 patients, 36 relapsed after SCT and for post-relapse therapy, received TKI therapy (n = 28), donor lymphocyte infusion (DLI, n = 1), immunosuppressant withdrawal (n = 5), follow-up loss (n = 1), and conservative care (n = 1). The cumulative incidence of relapse at 4 years was 29.6%. In analysis evaluating the predictive role of BCR-ABL1 transcript levels after SCT, MR4.5 at 3 months was associated with a lower relapse rate (13.3% ± 6.3% vs. 37.9% ± 6.8% at 4 years, p = 0.018) and higher EFS (80.0% ± 7.3% vs. 50.9% ± 6.9% at 4 years, p = 0.008), but did not influence OS (90.0% ± 5.5% vs. 81.1% ± 5.4%, p = 0.274) (Fig. 2). With additional information, among the 16 CP patients who had no available qRT-PCR data at 3 months after SCT, five patients relapsed at a median of 2.8 months (range, 1.3 to 24.7) after SCT and one patient died.
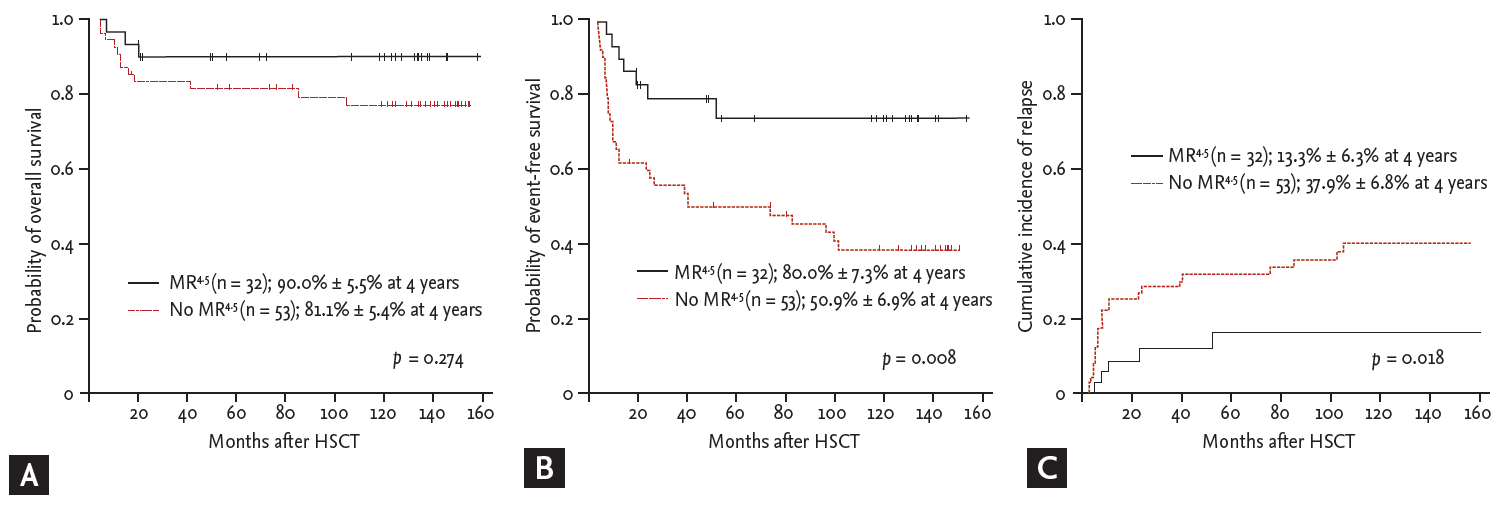
Transplantation outcomes according to BCR-ABL1 transcript levels at 3 months after allogeneic stem cell transplantation (SCT). Probabilities of (A) overall survival and (B) event-free survival, and (C) cumulative incidence of relapse according to MR4.5 at 3 months after SCT. HSCT, hematopoietic stem cell transplantation; MR, molecular response.
Multivariate analyses including potential clinical parameters
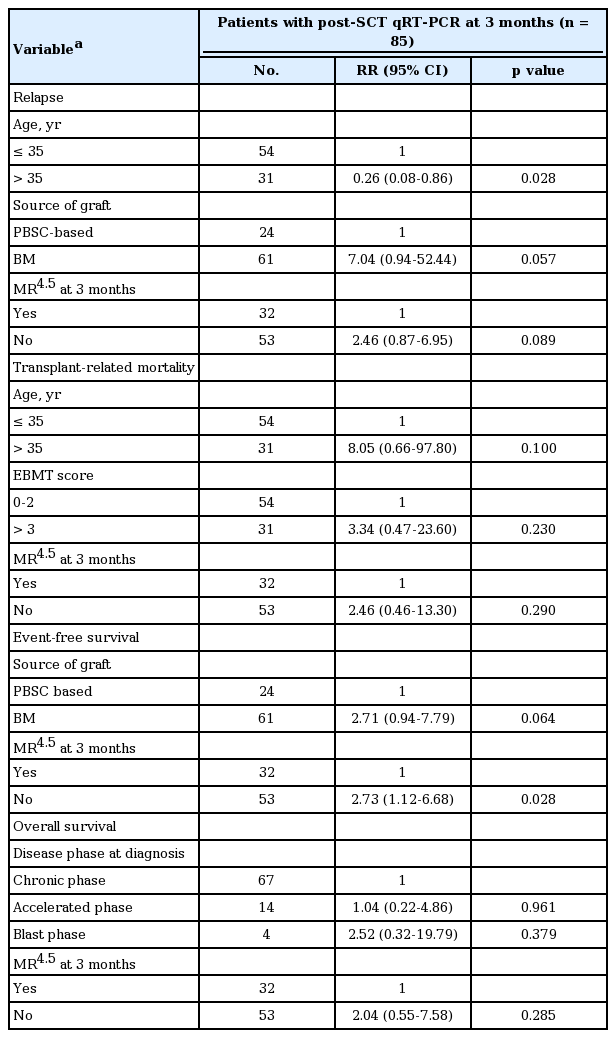
Potential predictive factors affecting relapse, EFS, and OS were assessed, including age, sex, Sokal risk, disease status at diagnosis and transplant, prior therapy before SCT, time from diagnosis to transplant, EBMT score, year of transplant, donor type, graft source, conditioning regimen, and GVHD prophylaxis (Table 3). In univariate analyses, younger age and BM graft source were potential risk factors for relapse. The BC at the time of diagnosis was a potential factor affecting OS, and the graft source was associated with EFS. In multivariate analyses including the potential variables affecting relapse and EFS respectively, MR4.5 at 3 months remained a predictive factor for higher EFS (risk ratio [RR], 2.73; 95% confidence interval [CI], 1.12 to 6.68; p = 0.028) and showed a trend for a lower relapse rate (RR, 2.46; 95% CI, 0.87 to 6.95; p = 0.089). However, MR4.5 at 3 months had no influence on OS (RR, 2.04; 95% CI, 0.55 to 7.58; p = 0.285) (Table 4).
DISCUSSION
In this study, 64% (23 of 36) of post-transplant relapse, defined as the ratio of BCR-ABL1 to ABL1 > 0.1% on the IS for two consecutive tests, occurred in the first 1 years of SCT. Therefore, we evaluated the predictive role of the BCR-ABL1 transcript levels at 1, 3, 6, and 9 months after SCT by ROC analysis (Supplementary Fig. 1), showing that 3-month data was used with maximized sensitivity in the prediction of patients at risk of relapse. Finally, we observed that MR4.5 at post-transplant 3 months was an early predictive factor for post-transplant relapse, and EFS in patients who underwent allogeneic SCT for CP CML. Several previous studies suggested that detection of BCR-ABL1 transcripts by qRT-PCR is associated with an increased risk of relapse [7,8]. Considering the limitation of differences in BCR-ABL1 kinetics in individual patients after allogeneic SCT, Asnafi et al. [8] evaluated the predictive role of BCR-ABL quantification at day 100 using qRT-PCR in 38 patients with > 1 year follow-up after conventional non-T-cell depleted SCT. They found that 14 patients with a high BCR-ABL/ABL ratio (≥ 10–4) had a higher relapse rate than 24 patients with a negative/low ratio (< 10–4).
In contrast, several studies suggested that an occasional positive test for BCR-ABL1 transcripts that was derived only from proliferating leukemia cells should not be interpreted as clinical relapse. Kaeda et al. [9] observed that BCR-ABL1 transcripts were detected at low levels in some patients for long periods after SCT without obvious progression. In their study, patients with a transcript level > 0.02% on three consecutive tests or > 0.05% on two consecutive tests were classified as having relapsed and were candidates for DLI. The other patients were classified into three categories: persistently negative for BCR-ABL1 transcripts, intermittently positive at a low level, and persistently positive at a low level. Only a minority of patients with fluctuating or persistently low levels of BCR-ABL1 transcripts satisfied their definitions of molecular relapse, whereas a majority of patients who satisfied their criteria for molecular relapse were likely to progress further [9]. Arpinati et al. [10] also reported results of 63 patients who underwent allogeneic SCT and had data from at least three qRT-PCR tests with a median follow-up of > 10 years. Eleven of the 63 evaluable patients never had BCR-ABL1 detectable transcripts, and none of these relapsed. Six of the 52 patients who had BCR-ABL1 transcripts detected at least once experienced post-transplant relapse. In their study, pre-emptive treatment was applied upon achieving transcripts levels in excess of 0.1% that were confirmed by the finding of Philadelphia chromosome-positive metaphases in the bone marrow [10]. The results of the above studies are consistent in that patients with persistent absence of detectable transcripts never relapsed and some patients who intermittently had low levels of transcripts inevitably relapsed. However, because the dose and duration of immune suppressive therapy (IST) can tailored in patients who are at high risk for post-transplant relapse, it is important to evaluate early predictors for post-transplant relapse. In this study, a total of 71 patients had paired qRT-PCR data of 3 and 6 months. Of the patients who did not achieve MR4.5 at 3 months, 57% of patients showed BCR-ABL1 transcript > 0.1% at 6 months and compared with 7% in MR4.5 group at 3 months, suggesting that a relatively fair number of these patients did eventually relapse. Moreover, the sensitivity of PCR technology has recently increased and the measurement of molecular responses has become standardized. Therefore, an early uniform BCR-ABL1 transcript cutoff on the IS for post-transplant relapse may provide additional information to guide clinical decisions on IST modulation.
The notion that the graft-versus-leukemia effect can play a role in maintaining remission was supported by the beneficial effects of DLI [15] and chronic GVHD [16]. The duration and withdrawal of immunosuppressive treatment are known to be important factors influencing the risk of relapse [17]. DLI has proved effective for the treatment of patients who relapse after allogeneic SCT, with stable responses of 60% to 70% in CP recurrence [18-21]. After the introduction of TKIs, the combination of IM with DLI became an option for achieving remission in patients with relapse [22] and many experiences of IM treatment for CML recurrence after SCT have been reported [5,6,23-25]. Wright et al. [6] showed the feasibility of TKIs including IM and/or DAS, with 64% of patients achieving molecular responses. Although frontline 2G-TKI therapy demonstrated faster and deeper responses than IM, the role of 2G-TKIs for management of post-transplant relapse is still limited by the lack of studies with a large series of patients. In our study, MR4.5 at 3 months was associated with a lower relapse rate and higher EFS, whereas there was no difference in OS. This might be because the MR4.5 cut-off at 3 months was determined in favor of sensitivity and not specificity, or because of the beneficial effect of TKI therapy for post-transplant relapse; 28 of the 36 relapsed patients received TKI therapy for post-transplant relapse and of them, 21 patients are alive in molecular remission, including UMRD in 16 patients, and seven patients died. Therefore, although the results of our study did not confirm that failure to achieve MR4.5 at 3 months indicates pre-emptive treatment with IM and 2G-TKIs, we can suggest that frequent molecular monitoring and IST modulation are required for patients with no reduction in BCR-ABL1 transcripts to these levels after SCT.
In our study, univariate analyses showed that use of PBSCs as a graft source was associated with a lower relapse rate and higher EFS, and that younger age was a potential factor for higher relapse. Considering that a PBSC source was used more frequently in the MR4.5 group, we performed multivariate analyses including potential variables affecting relapse and EFS, and found that MR4.5 at 3 months remained associated with higher EFS (p = 0.028) and showed a trend for a lower relapse rate (p = 0.089). In addition, the incidence of relapse was observed relatively lower in patients with cGVHD, compared with those of the patients without cGVHD (p < 0.001), supported by well-known graft versus leukemia effect associated with cGVHD [26,27]. In this study, the incidence of cGVHD was observed relatively lower in younger patients, which may be related with a higher relapse rate in younger age group. The significance of MR4.5 at 3 months held within a multivariate analysis’ model including cGVHD. Moreover, we observed no differences in aGVHD and cGVHD between the MR4.5 group and no MR4.5 group, suggesting that MR4.5 at 3 months can be used as an independent predictive value.
In conclusion, our data showed that MR4.5 at 3 months was associated with a lower relapse rate and higher EFS in patients who underwent allogeneic SCT for CP CML. This suggests that frequent molecular monitoring and intervention are required for patients who do not show a reduction in BCR-ABL1 transcripts to these levels after SCT. Additionally, the type of graft source was associated with relapse and EFS, and younger age was associated with relapse. However, future studies are needed to evaluate the use of TKIs in patients with a higher risk for relapse after SCT.
KEY MESSAGE
1. BCR-ABL1 transcripts (MR4.5) at post-transplant 3 months is predictive for relapse in allografted patients with chronic myeloid leukemia.
2. Immune suppressive therapy modulation based on the molecular monitoring is warranted.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (1020400) and the Korea Leukemia Bank (NRF-2013M3A9B8031236).
Supplementary Materials
Receiver operating characteristic (ROC) curves of BCR-ABL1 transcript level on the international scale (IS) at post-transplant (A) 1, (B) 3, (C) 6, and (D) 9 months. At post-transplant 1, 3, 6, and 9 months, 90, 85, 75, and 57 patients had available quantitative reverse transcriptase polymerase chain reaction data. AUC, area under the ROC curve; CI, confidence interval; MR, molecular response.