Serum vitamin D3 levels are not associated with thyroid cancer prevalence in euthyroid subjects without autoimmune thyroid disease
Article information
Abstract
Background/Aims
Previous studies have suggested that elevated serum vitamin D levels might protect against thyroid cancer. Elevated serum thyroid stimulating hormone levels and autoimmune thyroid disease (AITD) are suggested to be thyroid cancer promoting factors but have not been well controlled in previous studies. We designed the present study to evaluate whether serum vitamin D levels are associated with thyroid cancer in euthyroid patients with no clinical evidence of AITD.
Methods
This cross-sectional study included subjects who underwent routine health check-ups, including serum 25-hydroxy vitamin D3 (25(OH)D3) levels, anti-thyroid peroxidase antibody (TPO-Ab), and thyroid ultrasonography (US). Inclusion criteria were euthyroid, negative TPO-Ab, and no evidence of AITD by US findings. Thyroid cancer diagnoses were based on fine needle aspiration cytology and/or postsurgical histopathological findings.
Results
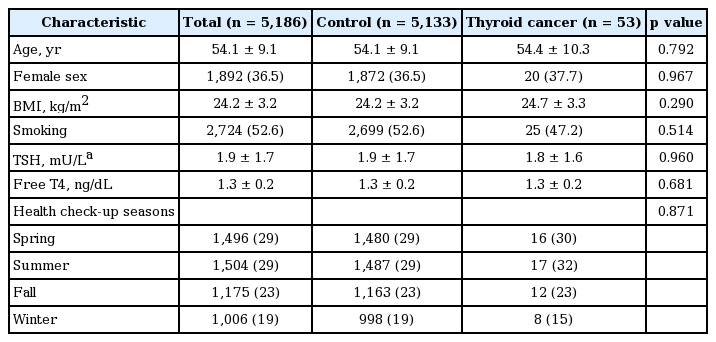
We enrolled 5,186 subjects (64% male, 37% female) in this study, including 53 patients (1%) with a diagnosis of thyroid cancer (33 males, 20 females). Mean 25(OH)D3 levels were similar between the thyroid cancer and control groups (p = 0.20). Subgroup analysis according to sex or seasonal variation also revealed no differences in 25(OH)D3 levels between the two groups. Based on the levels of 25(OH)D3, there was no significant difference in the prevalence of thyroid cancer; the prevalence was 0.71%, 0.94%, 1.40%, and 0.82% in the deficient, insufficient, sufficient, and excess groups, respectively (p = 0.64).
Conclusions
The levels of serum 25(OH)D3 are not associated with thyroid cancer prevalence in euthyroid subjects with no clinical evidence of AITD.
INTRODUCTION
Vitamin D is a pleiotropic hormone that affects both classical and nonclassical tissues, principally through the vitamin D receptor [1,2]. In addition to its role in calcium homeostasis, it has been associated with risk for several types of cancer, including colon, breast, and prostate cancer. It has received considerable recent attention as it exerts effects on cell proliferation, differentiation, apoptosis, and anti-angiogenesis [3-7]. Some experimental studies have suggested that vitamin D can protect against thyroid cancer [8-11]. However, clinical evidence for an association between lower serum vitamin D levels and an increased prevalence of thyroid cancer has been inconsistent [12-18].
The presence of autoimmune thyroid disease (AITD) and higher levels of serum thyroid stimulating hormone (TSH) have been considered to be risk factors for malignancies in thyroid nodules [19-21]. Previous studies have reported that the risk of thyroid malignancy in patients with nodular thyroid disease was increased by elevated serum TSH levels, even those within normal ranges, and higher serum TSH levels have also been associated with a more advanced stage of thyroid cancer [22]. Additionally, the presence of pathologically confirmed Hashimoto’s thyroiditis is associated with an increased risk of differentiated thyroid cancer, and has also been related to high TSH levels [23,24]. Therefore, the presence of AITD or elevated serum TSH levels may be serious confounding factors in evaluating the relationship between serum vitamin D levels and thyroid cancer. Previous studies did not control for these confounding factors, which might explain the inconsistent results obtained in these earlier reports.
This study aimed to evaluate the possible association between serum 25-hydroxy vitamin D3 (25(OH)D3) levels and thyroid cancer in a relatively large cohort using thyroid ultrasonography (US) screening. Importantly, we included subjects with normal thyroid function who showed no evidence of AITD according to serum anti-thyroid peroxidase antibody (TPO-Ab) and US findings.
METHODS
Subjects
We included subjects who underwent routine health check-ups at Asan Medical Center between 2008 and 2012. All subjects were interviewed and examined by physicians at the Health Promotion Center. Information about previous disease history, medication, and history of previous surgery was obtained from patients via self-administered questionnaires. Subjects were tested for TSH, free thyroxine (T4), serum 25(OH)D3, and TPO-Ab, and were simultaneously examined using thyroid US as previously reported [25].
Patients with positive TPO-Ab or US findings of heterogeneous parenchymal echogenicity that suggested AITD were excluded from this study. We also excluded subjects with abnormal thyroid, liver, or kidney function, a history of any thyroid disease or thyroid surgery, a family history of thyroid cancer, or any medication history that involved taking calcium or vitamin D supplements.
Seasons for the dates of health check-ups were categorized as spring (March to May), summer (June to August), fall (September to November), and winter (December to February) [26]. This study was approved by the Institutional Review Board of the Asan Medical Center, Seoul, Korea.
Laboratory measurements and US examinations
The serum 25(OH)D3 concentration was determined using the DIA source 25OH-Vit.D3-Ria-CT Kit (DIAsource ImmunoAssays S.A., Louvain-La-Neuve, Belgium; Cobra II Auto-γ Counting System, Packard Instruments, Downers Grove, IL, USA). We defined 25(OH)D3 levels as vitamin D deficient (< 10 ng/mL, n = 280 [5.4%]), insufficient (10 to 30 ng/mL, n = 3,285 [63.3%]), sufficient (30 to 50 ng/mL, n = 1,257 [24.3%]), or excess (> 50 ng/mL, n = 364 [7%]) [25,27]. The serum TPO-Ab concentration was determined using a BRAHMS anti-TPOn Radioimmunoassay (RIA) kit (Thermo Scientific, Darmstadt, Germany) with a functional sensitivity of 30 U/mL. A TPO-Ab level exceeding 60 U/mL was considered to constitute a positive TPO-Ab reading. Levels of serum TSH and free T4 were measured using the TSH-CTK-3 immunoradiometric assay kit (DiaSorinS.p.A, Saluggia, Italy) and free T4 RIA kit (Beckman Coulter/Immunotech, Prague, Czech Republic), respectively.
Identification of thyroid cancer
Among 5,186 subjects, 2,796 had cystic or solid nodules on thyroid US. Fine needle aspiration cytology (FNAC) was performed on 350 patients based on principles that have been described previously [28]. Among these patients, 26 were confirmed as thyroid cancer by histopathological results after surgery at the Asan Medical Center, including 23 classic PTC, two follicular variant PTC, and one medullary thyroid cancer. Additionally, 27 patients were diagnosed with ‘Bethesda VI: papillary thyroid carcinoma’ or ‘Bethesda V: suspicious for malignancy’ by FNAC but did not undergo surgery at Asan Medical Center. We included these two categories of patients in the thyroid cancer group, and overall there were 53 patients with thyroid cancer included in this study.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation and categorical variables are presented as number (%). Continuous variables were compared using the Student t test. Comparisons between groups of categorical variables were carried out using the Fisher exact test (two-sided). We performed univariate analysis of the association of thyroid cancer prevalence with various clinical parameters using a binary logistic regression model. The R software package version 3.0 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org) was used for statistical analysis. All p values were two sided and p < 0.05 was considered to denote a statistically significant difference.
RESULTS
Baseline clinical characteristics of control and thyroid cancer subjects
A total of 5,186 subjects (male 63.5%; female 36.5%) without evidence of AITD were eligible for this study. We diagnosed 53 patients (1.02%) as thyroid cancer (33 males and 20 females). The clinical characteristics of the thyroid cancer and control groups are described in Table 1. There was no significant difference in variables between the two groups.
Serum 25(OH)D3 levels in the thyroid cancer and control subjects
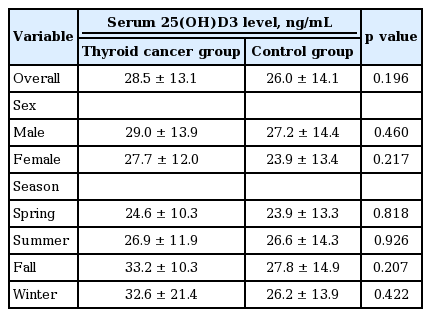
We compared 25(OH)D3 levels between the thyroid cancer and control groups (Table 2). There was no significant difference in 25(OH)D3 levels between the two groups (28.5 ± 13.1 ng/mL vs. 26.0 ± 14.1 ng/mL, p = 0.20). This pattern was similar in a subgroup analysis according to gender (male, 29.0 ± 13.9 ng/mL vs. 27.2 ± 14.4 ng/ mL, p = 0.46; female, 27.7 ± 12.0 ng/mL vs. 23.9 ± 13.4 ng/ mL, p = 0.22). To dissect the effects of seasonal variation on serum vitamin D levels, we also evaluated 25(OH)D3 levels during each season. Similarly, in all seasons, there was no significant difference in 25(OH)D3 levels between thyroid cancer and control patients (Table 2).
The prevalence of thyroid cancer according to the serum 25(OH)D3 levels
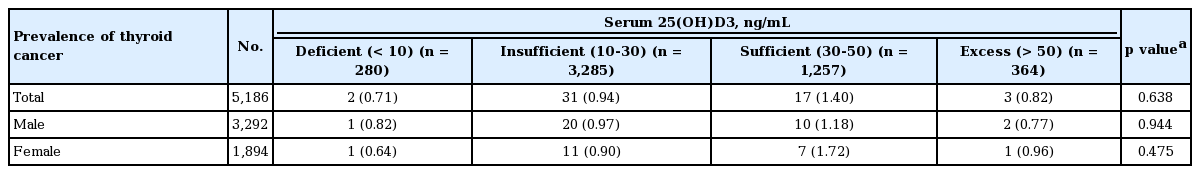
We classified study subjects into four groups according to 25(OH)D3 levels as follows: deficient (< 10 ng/mL), insufficient (10 to 30 ng/mL), sufficient (30 to 50 ng/mL), and excess (> 50 ng/mL). As shown in Table 3, there was no significant difference in the prevalence of thyroid cancer among the four groups; the frequency of thyroid cancer was 0.71%, 0.94%, 1.40%, and 0.82% in the deficient, insufficient, sufficient, and excess groups, respectively (p = 0.64). Subgroup analysis based on gender did not show any differences between the serum 25(OH)D3 groups.
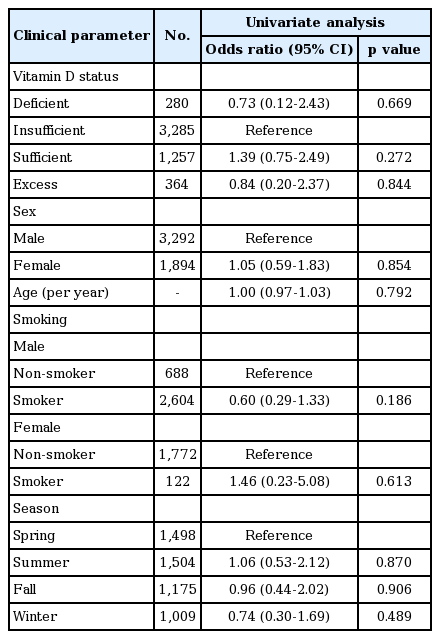
We performed univariate analysis to evaluate possible associations between thyroid cancer prevalence and various clinical parameters using a binary logistic regression model. There was no clinical parameter that showed a statistically significant correlation with thyroid cancer prevalence (Table 4).
DISCUSSION
The potential association between serum vitamin D levels and thyroid cancer has been inconclusive based on previous studies [29]. In some reports, lower levels of serum vitamin D in thyroid cancer patients or a higher malignancy rate in vitamin D-deficient individuals were observed [12-15], and there was a correlation between serum vitamin D levels and tumor size or staging [14,15]. However, others have detected no significant difference in serum vitamin D levels between thyroid cancer and control groups [16-18].
These previous studies had two important limitations. First, serum TSH levels and the presence of AITD could be confounding factors in evaluating the association between serum vitamin D levels and thyroid cancer. However, it is unclear that they completely controlled for these confounding factors. Therefore, our cross-sectional study included subjects with normal thyroid function and excluded subjects with any evidence of AITD; we used serum TPO-Ab and thyroid US findings to completely control for this confounding factor. When we analyzed patients with AITD separately, there was no significant association between serum 25(OH)D3 level and thyroid cancer prevalence (data not shown). The second limitation of previous studies is reverse-causation bias. Poor health conditions could reduce outdoor activities, limit sun exposure, and alter the diet of study subjects resulting in lower serum vitamin D levels [29]. Our study included relatively healthy subjects in routine health check-ups and newly diagnosed thyroid cancer patients after thyroid US screening.
The incidence of thyroid cancer, especially PTC, has been progressively increasing worldwide, and in Korea the incidence was among the highest in 2008 [30,31]. According to the 2011 Korean Cancer Registry, its annual incidence rate was 81.0 per 100,000 persons (men, 27.9; women, 134.1), which represented 17.8% of all cancers diagnosed in South Korea in 2011. The relative ease of thyroid cancer detection using high-resolution US may have contributed the more frequent diagnosis of thyroid cancer; however, it cannot fully explain the increased occurrence and there likely has been a real increase in thyroid cancer incidence [32]. To identify the cause of the increase in thyroid cancer incidence, diverse causes, including genetic and environmental factors, have been proposed [33].
A high prevalence of vitamin D deficiency and insufficiency has been reported worldwide [34]. In Korea, vitamin D deficiency has become a public health problem not only in elderly persons, but also in younger individuals because of changes in lifestyle and occupations. The prevalence of vitamin D deficiency, defined as serum 25(OH)D3 levels below 30 ng/mL, was 86.8% in men and 93.3% in women [35]. Various experimental studies have suggested that vitamin D has a strong likelihood of exerting anti-cancer bioactivities [1]. The vitamin D receptor is expressed in most human tissues. Studies using in vitro and in vivo experimental models suggested that 1,25-dihydroxy vitamin D3 promotes cell differentiation, inhibits cancer cell proliferation, and exhibits anti-inflammatory, pro-apoptotic, and anti-angiogenesis properties [1]. However, in human cancer prevention, vitamin D has not been widely accepted in variable human cancers, including thyroid cancer [29]. In this study, we measured serum 25(OH)D3 levels in thyroid cancer and control patients after US screening in a large cohort with normal thyroid function and without any evidence of AITD. Our present findings support neither protective roles nor adverse effects of vitamin D on thyroid cancer development. Even, some studies have raised concerns about a potential association between increased risk for selected cancers and high levels of serum vitamin D [1,36].
This study had several limitations. First, it employed a cross-sectional design. In this study, thyroid cancer was identified as part of routine clinical practice base on the sonographic finding and size of thyroid nodule. So we could not totally exclude the possibility of false negative patients in control group. Second, there was a possibility of selection bias because we enrolled subjects who visited a Health Promotion Center for routine health check-ups. Third, there was a limitation in confirming the final histopathological results in some thyroid cancer patients because these patients had a confirmation of thyroid cancer based on FNAC results and most of them were treated at another hospital. Lastly, ultrahigh performance liquid chromatography-tandem mass spectrometry was considered to be the gold standard for measuring 25(OH)D3, but this assay was not routinely used by reference laboratories for clinical samples [37]. However, our study had the advantage of including a relatively large number of subjects to evaluate the prevalence of thyroid cancer, and about 1% of the subjects were classified in the thyroid cancer group. We only included newly diagnosed thyroid cancer after US screening and included subjects with normal thyroid function and no evidence of AITD to control for this important confounding factor. Thyroid cancer incidence in another large cohort of vitamin D3 deficiency without AITD needs to confirm our findings.
In conclusion, there was no significant association between serum 25(OH)D3 levels and thyroid cancer in our US screened cohort with normal thyroid function and no clinical evidence of AITD.
KEY MESSAGE
1. Vitamin D is a pleiotropic hormone and its elevated serum levels were suggested to protect against thyroid cancer.
2. The presence of autoimmune thyroid disease (AITD) and higher levels of serum thyroid stimulating hormone have been considered to be risk factors for malignancies in thyroid nodules.
3. In our study, there was no significant association between serum 25(OH)D3 levels and thyroid cancer in our ultrasonography-screened cohort with normal thyroid function and no clinical evidence of AITD.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This paper was supported by Bumsuk Academic Research Fund in 2014.