Treadmill exercise-induced E/e’ elevation as a predictor of cardiovascular event in end-stage renal disease on peritoneal dialysis
Article information
Abstract
Background/Aims
Diastolic dysfunction is associated with cardiovascular (CV) events in end-stage renal disease (ESRD) on continuous ambulatory peritoneal dialysis (CAPD). However, conventional measurement of LA volume and E/e’ using Doppler echocardiography has been limited to predict CV events in patients with ESRD on CAPD.
Methods
From September 2007 to September 2008, 30 consecutive patients with exertional dyspnea in ESRD on CAPD and normal systolic function was prospectively enrolled and underwent laboratory testing, coronary angiography, and treadmill exercise stress echocardiography (TESE). We divided the patients according to the presence of exercise-induced change of E/e’ tissue Doppler and investigated whether this factor predicted CV events in ESRD on CAPD.
Results
Mean CAPD duration of all patients (70% male; mean age, 49 years) was 12 months. Patients with exercise-induced elevated E/e’ (n = 12, 40%) and non-elevated E/e’ (n = 18, 60%) demonstrated similar baseline characteristics. Exercise-induced elevated E/e’ was predictable (cut-off value 14%, sensitivity 63%, and specificity 95%), with a hazard ratio of 1.13 (confidence interval, 1.03 to 1.24; p = 0.005), and significantly associated with CV events compared to the non-elevated E/e’ group (log-rank, p = 0.007).
Conclusions
Exercise-induced elevated E/e’ determined using TESE might be feasible to predict CV events in patients with ESRD on CAPD.
INTRODUCTION
Diastolic dysfunction (DD) with normal systolic function is associated with increased cardiovascular (CV) events in patients with end-stage renal disease (ESRD) [1]. However, DD in ESRD may be under-diagnosed due to the changeable volume status and hemodynamic parameters in clinical settings [2,3]. The ratio of early diastolic mitral inflow velocity to early diastolic mitral annulus velocity (E/e’) determined by using echocardiography has been used as a marker of DD [4]. In addition, E/e’ by Doppler was also demonstrated to be a prognostic factor for CV mortality which is extended to be applicable in the real disease as previously reported [4-8]. However, in ESRD on continuous ambulatory peritoneal dialysis (CAPD), the value of the exercise-induced E/e’ as a predictor for CV event has not been reported yet. Therefore, we investigated the change of E/e’ during treadmill exercise stress echocardiography (TESE) and its association with CV events in ESRD patients on CAPD.
METHODS
Patients
A total of 40 consecutive patients with exertional dyspnea who were treated with CAPD due to ESRD at Eulji University Hospital, Daejeon, South Korea between September 2007 and December 2008 were prospectively enrolled in this study. The study protocol adhered to the Declaration of Helsinki and was approved by the Institutional Review Board of Eulji University Hospital. Written informed consent was obtained from all patients. All patients underwent routine baseline chest radiograph, laboratory testing, electrocardiography, TESE, and coronary angiography. Among these patients, 10 among all patients were excluded for the following reasons: peritoneal dialysis duration < 3 months (n = 1), left ventricular (LV) systolic dysfunction (ejection fraction [EF] < 55, n = 5), severe valvular disease (n = 1), and significant coronary artery disease (n = 3).
Data collection
Patients’ baseline and clinical characteristics such as age, sex, and comorbid conditions were recorded. At the same time, laboratory parameters such as hemoglobin serum albumin, blood urea nitrogen, creatinine, calcium, phosphorus, intact parathyroid hormone, and C-reactive protein were measured. A peritoneal equilibrium test (PET) was conducted, and dialysis adequacy and residual renal function were measured within 2 months after the initiation of CAPD. Equilibration ratios were measured with a standardized PET protocol that involved a 2-L 4.25% dextrose dwell with dialyzed samples taken at 0, 2, and 4 hours and a plasma sample at 2 hours.
Creatinine and urea nitrogen were measured in blood, urine, and drained dialysate. The residual glomerular filtration rate was calculated as the mean of urinary creatinine and urea clearances standardized to 1.73 m2 body surface area (BSA). Weekly Kt/V urea was calculated as the ratio of urinary and 24-hour dialysate urea clearance to total body water [9].
Coronary angiography
An invasive coronary angiogram was performed subsequently for evaluation of exertional dyspnea. The conventional Judkins’ technique was used with at least four views of the left and two views of the right coronary artery. Lesions were examined in orthogonal views, and severity of luminal stenosis was determined by the consensus of two experienced cardiologists who performed quantitative assessment of stenosis severity using standard techniques.
Echocardiography
Echocardiographic examinations were performed using an imaging protocol according to the American Society of Echocardiography recommendations [10,11]. This examination was performed in CAPD patients with an empty abdomen. Standard echocardiography analysis included two-dimensional, M-mode, and Doppler flow measurements and used commercially available ultrasound equipment (Acuson Sequoia, Siemens Medical Solutions, Mountain View, CA, USA; GE Vivid 7 and 9, GE Healthcare, Wauwatosa, WI, USA). The LV systolic function was determined from the LVEF using a modified biplane Simpson’s method from apical two- and four-chamber views and systolic dysfunction was defined as EF < 55%. The LV mass was calculated by the method described by Devereux and Reichek [12]. The left ventricular mass index (LVMI) was calculated by dividing LV mass by BSA, and LV hypertrophy was defined as a LVMI > 131 g/m2 for men and > 100 g/m2 for women [13]. The left atrial (LA) volume was assessed by the biplane area-length method from apical four- and two-chamber views, with measurements obtained in end systole from the frame preceding mitral valve opening, and the LA volume was indexed for BSA. According to population-based studies, a value of 32 mL/m2 was considered the upper limit of normal left atrial volume index (LAVI) [14]. Mitral inflow was assessed with pulse-wave Doppler echocardiography from the apical four-chamber view. The Doppler beam was aligned parallel to the direction of flow, and a 1- to 2-mm sample volume was placed between the tips of the mitral leaflets during diastole. From the mitral inflow profile, peak E-wave velocity and its deceleration time, peak A-wave velocity, and the isovolumetric relaxation time were measured, and Doppler tissue imaging of the mitral annulus was also obtained. From the apical four-chamber view, a 1- to 2-mm sample volume was placed in the septal mitral annulus. An early mitral inflow velocity to peak mitral annulus velocity (E/e’) ratio > 15 was regarded as indicating abnormally elevated LV filling pressure [6,15].
Treadmill exercise stress echocardiography
All patients underwent TESE with standard protocols (Bruce and modified Bruce) chosen as previously reported [16,17]. Post-exercise echocardiographic images were acquired within 30 to 60 seconds after the termination of treadmill exercise. Treadmill exercise was conducted according to the Bruce protocol in 20 patients, and modified Bruce protocol in 10 patients. Patients were asked to stop taking calcium channel blocker or β-blockers at least 12 to 24 hours prior to TESE. All patients exercised to exhaustion, irrespective of the achieved heart rate, and were asked not to hold on to the handrails. However, the test was terminated prematurely if patients developed severe chest pain, symptomatic hypotension, systolic blood pressure > 250 mmHg, significant arrhythmia, severe ST segment changes, or per the patient’s request. Patients were monitored for heart rate, blood pressure, rhythm, symptoms, electrocardiogram changes, and rate of perceived exertion (on a 1 to 10 scale, with 10 indicating maximum exertion) at rest and at every stage of the exercise protocol. Exercise capacity in metabolic equivalents (METs; 1 MET = 3.5 mL/kg/min of oxygen consumption) was estimated on the basis of the protocol, speed, and grade achieved [18].
Clinical follow-up and cardiovascular events
Patients were divided into two groups: exercise-induced elevated E/e’ (n = 12) and non-elevated E/e’ (n = 18) by comparison baseline E/e’ with E/e’ after treadmill exercise in each patient. These groups were followed for CV events including CV death and heart failure (HF) hospitalization, which were monitored regularly on an outpatient basis.
Statistical analysis
Statistical analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA) and MedCalc Software (Acacialaan, Ostend, Belgium). Continuous data were expressed as mean ± standard deviation, and categorical data were expressed as number (percentage). The two groups were compared by t test or chi-square test. The relationship between paired variables was analyzed using the Pearson sample correlation coefficient. Cumulative survival curves were generated by the Kaplan-Meier method, and between-group survival was compared by the log-rank test. Univariate analysis included clinical, echocardiographic and hemodynamic variables, and variables with a p < 0.15 in the univariate analysis were entered into the multivariate analysis. The independent prognostic value for CV event was analyzed by multiple Cox regression analysis. Hazard ratio (HR) and 95% confidence interval (CI) were calculated with the use of the estimated regression coefficients and standard errors. The predictive value for CV event was analyzed by receiver operating characteristic (ROC) curve analysis with calculated area under the ROC curve (AUC). All probabilities were two tailed, and the threshold for significance was set at 0.05.
RESULTS
Baseline and clinical characteristics
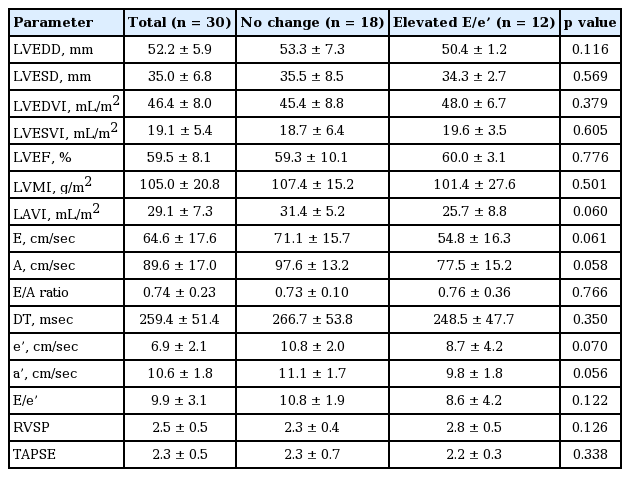
The comparison of baseline and clinical characteristics between the groups defined by elevated E/e’ were shown in Table 1. Patients’ mean age was 49 ± 10 years, and most (70%) were male. The primary etiology of ESRD was hypertension, and 73% of ESRD patients were also taking anti-diabetic medication (Table 2). No previous cerebrovascular disease exists in all patients. Body mass index, diastolic and systolic blood pressure, and heart rate were similar between the two groups. In addition, there was no significant difference in any baseline parameters except for triglyceride, including blood urea nitrogen, creatinine, high density lipoprotein cholesterol, low density lipoprotein cholesterol, total cholesterol, parathyroid hormone, calcium, total protein, albumin, cardiac biomarkers, and C-reactive protein (Table 2).
Treadmill exercise stress echocardiography
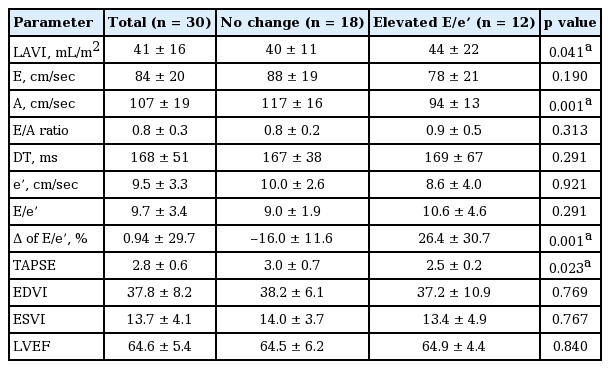
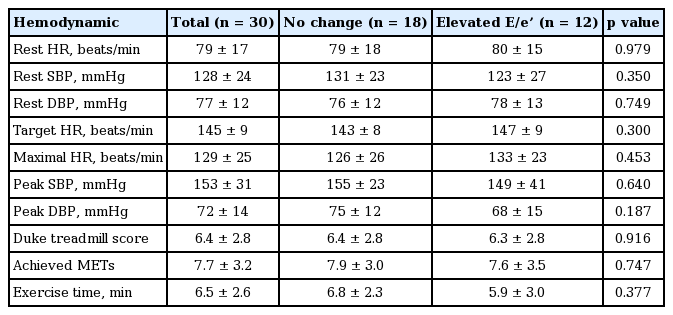
The baseline echocardiographic measurements and changes in these parameters after exercise are presented in Tables 3 and 4. In the comparison of echocardiographic findings including LVMI, LVEF, and LAVI were similar between the two groups. Hemodynamic parameters of TESE are shown in the Table 5. The average exercise capacity and exercise duration in all patients were 7.7 MET and 6.5 minutes. Heart rate and blood pressure were similar between the groups both at rest and upon exercise-induced elevation. In the exercise condition, diastolic A velocity and tricuspid annular plane systolic excursion were higher in the non-elevated E/e’ group and lower in the elevated E/e’ group, with a statistically significant difference between the groups, and LAVI was lower in the non-elevated E/e’ group and higher in elevated E/e’ group. Interestingly, the absolute value of post-exercise E/e’ was not different between two groups, but delta E/e’ was significantly elevated in the elevated E/e’ group which is more important parameter to predict CV events.
Elevated E/e’ as a predictor of clinical outcomes
During follow-up of 35 ± 6 months, six of 30 patients (20%) experienced CV events including cardiac death (n = 4; suspicious sudden cardiac death [n = 3] and suspicious decompensated HF [n = 1], 13%) or HF hospitalization (n = 2, 6%). The event rate of CV death was higher in the elevated E/e’ group than in the non-elevated E/e’ group (p < 0.001). Baseline and post-exercise E/e’ were not significantly different between CV event-free and CV events group but exercise-induced change of E/e’ was significantly higher in CV events group (Supplementary Table 1). The ROC curve for CV events indicated that elevated E/e’ at a cut-off value of 14% was predictive of CV event with a sensitivity of 64% and specificity of 95% (AUC = 0.861 [0.686 to 0.959] with p < 0.001) (Fig. 1). Kaplan-Meier curve also representing a greater risk of CV events in the elevated E/e’ group (log-rank test, p = 0.007) (Fig. 2). In the Cox proportional hazards model, conventional risk factors of ESRD including age, sex, hypertension, diabetes, hemoglobin, uric acid, brain natriuretic peptide, EF, and LAVI were analyzed. In the multivariate analysis, exercise-induced elevated E/e’ was an independent predictor of CV events (HR, 1.135; 95% CI, 1.039 to 1.241; p = 0.005) (Table 6).

Receiver operating characteristic analysis representing exercise-induced E/e’ for prediction of cardiovascular events.
DISCUSSION
Our study suggests that exercise-induced elevated E/e’ is feasible for predicting the occurrence of CV events and might be a useful predictor of CV events in ESRD on CAPD who have normal systolic function.
A previous study demonstrated that Doppler parameters can provide information about LV filling pressure [19]. However, controversy remains concerning which parameters, such as E/e’ or LAVI, provide predictive information for DD and which parameter is a more powerful prognostic indicator of CV events. Integrating multiple Doppler-derived indices can be useful for the diagnosis and grading of DD. Therefore, integration of the E/A, E/e’, and LAVI has been used by international consensus and recommendation to classify DD [20]. This grading DD was known to predict CV events only in the general population. However, recently, DD-associated CV events have also been investigated in ESRD [21,22]. These findings suggest that an assessment of DD using multiple echocardiographic indices can increase the predictive power for future CV events in ESRD patients. Recent reports have also shown that Doppler-derived E/e’ may diagnose DD and predict CV events [4,23]. In the chronic kidney disease of stages III to V with DD, Kim et al. [8] also demonstrated that 5-year mortality rate was 60% and the increased E/e’ significantly associated with CV events. In addition, Doppler-derived LA volume was reported to be significantly correlated with CV events in ESRD [24,25]. However, Doppler-derived E/e’ and LAVI can vary according to the systemic volume status and heart rate in ESRD. Therefore, we tried to focus that the CAPD patients without CV disease was more stable than hemodialysis patients in the clinical status, which may not be influenced by several different factors such as the change of serum ionized calcium concentration, renal anemia, the presence of an arteriovenous fistula with high flow rate, sympathetic hyperactivity, increased oxidative stress, LA volume during hemodialysis treatment, LV compliance, and disease of smaller resistance vessels [14,26-29].
Ha et al. [30] and Bergeron et al. [31] demonstrated that patients with unexplained dyspnea have a high likelihood of CV events, and that exercise echocardiography provides independent information to identify patients at risk and evaluate diastolic HF in the general population. In addition, Wang et al. [21] also demonstrated that Doppler-derived E/e’ estimation provided independent prognostic value for predicting long-term CV events in ESRD patients. Aljaroudi et al. [32] found that hypertrophic cardiomyopathy patients with worse DD had impaired exercise tolerance, with an odds ratio of 3.0 and suggested that TESE should remain a valid tool in the hypertrophic cardiomyopathy irrespective of baseline DD.
In our study, we selected stable CAPD patients with exertional dyspnea and minimized influencing factors such as coronary artery disease, cardiomyopathy, valvular heart disease, and LV dysfunction. We hypothesized that stable CAPD with exertional dyspnea may be correlated with the change of E/e’ on TESE and divided patients into two groups accordingly: exercise-induced elevated E/e’ and non-elevated E/e’ groups. With prospective enrollment, the baseline and clinical characteristics were similar between the two groups, including baseline laboratory and echocardiography findings. Comparison of exercise-induced elevated E/e’ and non-elevated E/e’ revealed no significant differences except A velocity which may be explained by change of LA function on the stress exercise. In addition, Doppler-derived E’ velocity in the elevated E/e’ group was lower than that in the non-elevated E/e’ group without statistically significance which explained by stress exercise-related influences on diastolic function such as worsening of LV stiffness and LV diastolic filling pressure. In addition, the change in LAVI significantly differed between the elevated E/e’ and non-elevated E/e’ groups, as previously reported [24]. In the ROC curve analysis, exercise-induced elevated E/e’ demonstrated predictability for CV events, with sensitivity of 64%, specificity of 95% (cut-off value 14%), and AUC = 0.861 (0.686 to 0.959) with p < 0.001, as shown in Fig. 1. In multiple Cox regression analysis, exercise-induced elevated E/e’ was the strongest predictor for CV events (HR, 1.13; 95% CI, 1.03 to 1.24; p = 0.005) (Table 4) and significantly associated with CV events compared to the non-elevated E/e’ group (log-rank, p = 0.007) (Fig. 2).
This study has some limitations. First, we did not evaluate the exact volume status of study patients before and after CAPD with an objective tool, and it is possible that some patients will have experienced significant volume overload even after optimal peritoneal dialysis treatment. Second, the results are based on a small number of patients, and more patients should be examined in future studies. However, this study used a well-controlled prospective design in the specific ESRD on CAPD. Third, TESE results can show interobserver and intraobserver variability. Forth, definition of increased E/e’ may be ambiguous, we focused on the arbitrary absolute value delta of E/e’ after exercise compared with prior to exercise using by ROC curve analysis. Therefore, we suggested that increased delta 14% may be statistically significant cut-off value to discriminate the prediction of CV events in our study. However, large population study should be needed to confirm its value. Finally, the study observation of clinical follow-up should be sufficient to demonstrate CV events; but it was not enough to assess long-term effects. However, it may show the relative trend of CV events in ESRD on CAPD.
In conclusion, this study is the first to demonstrate that CAPD with exertional dyspnea who demonstrate exercise-induced elevated E/e’ on TESE are at higher risk for the occurrence of CV events. In such patients, exercise-induced elevated E/e’ by Doppler was feasible and could hold prognostic value for the occurrence of CV events in the future.
KEY MESSAGE
1. Treadmill exercise stress echocardiography was feasible for evaluation of exertional dyspnea with normal systolic function in the end-stage renal disease (ESRD) with continuous ambulatory peritoneal dialysis (CAPD).
2. Exercise-induced elevated E/e’ by Doppler implicates prognostic value for the occurrence of cardiovascular events in the ESRD with CAPD.
Notes
This research was supported by Handok Pharmaceutical Co., Ltd., Republic of Korea.