Analysis of computed tomographic findings according to gastroesophageal flap valve grade
Article information
Abstract
Background/Aims
The gastroesophageal junction is an important barrier against gastroesophageal ref lux. Endoscopic grading of gastroesophageal f lap valve is simple, reproducible, and may predict reflux activity. We investigated the correlation between gastroesophageal flap valve grade and the gastroesophageal junction’s structural properties using abdominal computed tomography.
Methods
A total of 138 patients with early gastric cancer who underwent both pre-treatment esophagogastroduodenoscopy and water-distended stomach two-phase computed tomography were enrolled. Endoscopic gastroesophageal f lap valve grade and abdominal computed tomography findings were analyzed to assess anatomical factors including the gastroesophageal junction and related organs.
Results
The angle of His increased significantly with gastroesophageal flap valve grade (grade I, 65.2˚ ± 19.6˚; grade II, 66.6˚ ± 19.8˚; grade III, 76.7˚ ± 11.9˚; grade IV, 120.0˚ ± 30.3˚; p < 0.001), as did the size of the diaphragmatic hiatus (grade I, 213.0 ± 53.8 mm2 ; grade II, 232.6 ± 71.0 mm2 ; grade III, 292.3 ± 99.2 mm2 ; grade IV, 584.4 ± 268.3; p < 0.001). The length of the abdominal esophagus decreased as gastroesophageal flap valve grade increased (grade I, 34.6 ± 5.8 mm; grade II, 32.0 ± 6.5 mm; grade III, 24.6 ± 7.8 mm; grade IV, –22.6 ± 38.2 mm; p < 0.001). There was no significant relationship between gastroesophageal flap valve grade and visceral and subcutaneous fat areas (p = 0.877 and p = 0.508, respectively).
Conclusions
Endoscopic grading of the gastroesophageal flap valve is well correlated with anatomical changes around the gastroesophageal junction on abdominal computed tomography, and it can provide useful information about the anti-reflux barrier.
INTRODUCTION
The gastroesophageal junction (GEJ) is important for preventing gastroesophageal reflux [1]. The normal anti-reflux barrier at the GEJ is predominantly maintained by the intrinsic lower esophageal sphincter (LES) and extrinsic compression of the LES by the crural fibers of the diaphragm [1]. In addition, the flap valve at the GEJ also contributes to the barrier function [1,2]. Since evidence of a flap valve at the GEJ was provided from cadaver studies [3], an extension of this observation has been published regarding the gastroesophageal flap valve (GEFV) [4]. The acute angle of His is primarily maintained by the collar sling musculature of the gastric cardia [5], and the intraluminal extension of the angle of His creates the GEFV. The GEFV, as viewed with a retroflexed endoscope, is a 180° musculomucosal fold that allows for one-way passage of ingested contents into the stomach and prevents reflux [6,7]. The GEFV grading system is an easy and simple tool that provides useful information about patients with suspected gastroesophageal reflux during endoscopy [4,8,9]. Moreover, the clinical importance of the GEFV is that an increased GEFV grade is associated with an increased prevalence of reflux esophagitis, Barrett’s esophagus, abnormal esophageal acid exposure, and the prevalence of a mechanically defective sphincter [8-10].
Several studies have shown morphological changes at the GEJ in patients with gastroesophageal reflux disease (GERD) [11,12] and have provided strong evidence for the structural components of reflux protection by the ‘flap valve’ mechanism using magnetic resonance imaging (MRI) and computed tomography (CT) [11-14]. A recent study that analyzed CT images showed a more frequent occurrence of hiatal hernia, a greater angle of His, and a larger diaphragmatic hiatus in patients with severe reflux esophagitis [12].
Based on the results of these studies, patients with severe reflux esophagitis are expected to show anatomical defects around the GEJ, and early surgery such as fundoplication can be considered in these patients. Likewise, an abnormal GEFV is expected to be related to anatomical changes, and grading of the GEFV may be helpful in selecting candidates for surgery. In this regard, a previous study reported that patients with grade IV GEFV underwent surgery for either failed medical management or disease complications more frequently than did patients with lower GEFV grades [15]. However, there is still lack of data regarding any correlation between GEFV grade and anatomy around the GEJ. In the present study, we analyzed abdominal CT images to assess anatomical factors, including the GEJ and related organs, such as the diaphragm and fat, according to GEFV grade.
METHODS
Patients
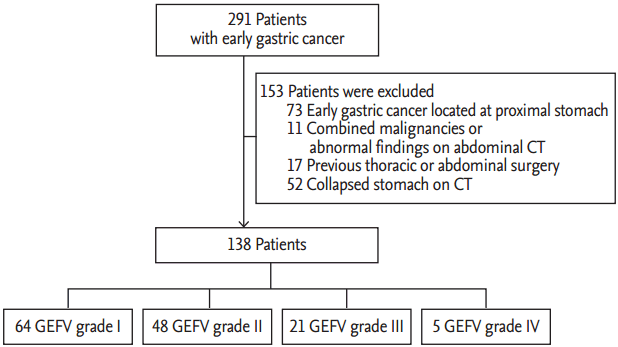
To compare the GEFV grade with abdominal CT findings from around the GEJ, we planned to include patients who had undergone both esophagogastroduodenoscopy (EGD) and abdominal CT around the same time. From January 2012 to December 2012, a total of 291 patients diagnosed with early gastric cancer (EGC) at Pusan National University Hospital (Busan, Korea) were enrolled. All patients had undergone both EGD and abdominal CT within 1 month of each other. To minimize the impact caused by EGC or other conditions, we excluded patients with EGC located at the proximal stomach (n = 73), with combined malignancies or abnormal findings in the lower esophagus and stomach on abdominal CT (n = 11), or with a history of previous thoracic or abdominal surgery (n = 17). In addition, we excluded 52 patients who did not undergo water-distended stomach two-phase CT, because the stomach was collapsed in these patients. Finally, 138 patients were included in the analysis of endoscopic and abdominal CT findings (Fig. 1). The study’s protocol was reviewed and approved by the Institutional Review Board of the Pusan National University Hospital (E-2015029).
Endoscopic assessment
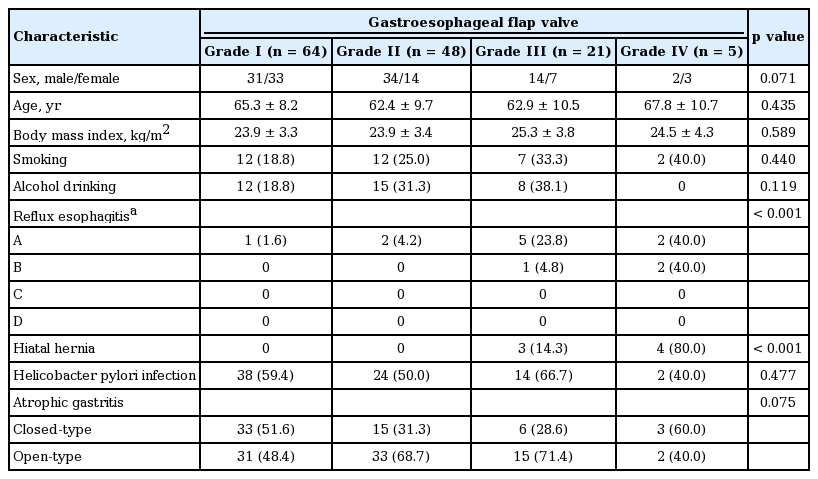
The presence or absence of reflux esophagitis and hiatal hernia and the grades for GEFV and atrophic gastritis were prospectively determined during endoscopic examination by one expert endoscopist (G.H.K.). All the endoscopic images of enrolled patients were independently reviewed again by two gastroenterologists (H.K.J. and G.H.K.) without any information regarding the study subjects. If the results did not match, those data were adjusted through a discussion. Gastric antral and corpus biopsy samples were taken for the detection of Helicobacter pylori infection by rapid urease test.
Reflux esophagitis
If esophagitis was present, it was graded according to the Los Angeles classification system [16].
Hiatal hernia
Hiatal hernia was defined as a circular extension of the gastric mucosa above the diaphragmatic hiatus that was greater than 2 cm in axial length.
Gastroesophageal flap valve
The GEJ was viewed using a retroflexed endoscope during gastric inflation. GEFV was largely classified into two groups [7,9]: normal GEFV (grades I and II) and abnormal GEFV (grades III and IV). The grading system for GEFV consisted of the following four grades (Fig. 2) [4].

(A) Grade I: the prominent fold of tissue along the lesser curvature of the stomach is closely apposed to the endoscope. (B) Grade II: the fold is present, but there are periods of opening and rapid closing around the endoscope. (C) Grade III: the fold is not prominent, and the endoscope is not gripped tightly by the ridge. (D) Grade IV: there is no fold, and the lumen of the esophagus gapes open, allowing the squamous epithelium below to be seen.
Grade I: a prominent fold of tissue along the lesser curvature is closely apposed to the endoscope.
Grade II: the fold is present but there are periods of opening and rapid closing around the endoscope.
Grade III: the fold is not prominent, and the endoscope is not gripped tightly by the ridge.
Grade IV: there is no fold, and the lumen of the esophagus gapes open, allowing the squamous epithelium below to be viewed.
Atrophic gastritis
The grade of atrophic gastritis was assessed endoscopically using the atrophic pattern system described by Kimura et al. [17]. This classification system divides the extent of atrophy into the closed and open types. In the closed-type, the atrophic border remains on the lesser curvature of the stomach, while in the open-type, the atrophic border no longer exists on the lesser curvature but extends along the anterior and posterior walls of the stomach.
Abdominal CT technique
All multi-detector row computed tomography (MDCT) was performed according to the protocol used by our department. CT images were obtained using several scanners: a 16-MDCT scanner (Sensation 16, Siemens Medical Systems, Erlangen, Germany) for 11 patients, a 64-MDCT scanner (Somatom Definition, Siemens Healthcare, Forchheim, Germany; and Discovery 750HD, GE Healthcare, Milwaukee, WI, USA) for 109 patients and a 128-MDCT scanner (Somatom Definition AS+, Siemens Healthcare) for 18 patients. After patients had fasted for 8 hours, CT scanning was performed while patients were in a near-supine position with the head supported at 15º by a pillow placed under the back. All patients were required to drink 500 mL of water approximately 15 minutes before the examination and an additional 500 mL immediately prior to the study. Water was used as a negative contrast agent to distend the stomach and improve visualization of the gastric wall. In all patients, intravenous injection of nonionic iodinated contrast (Ultravist, Bayer-Schering Pharma, Seoul, Korea) was administered at a dosage of 1.5 mL/kg of body weight at an injection rate of 3 mL/sec using an automated pump through a 18- to 20-gauge intravenous catheter placed in the antecubital vein.
Two sets of two-phase contrast-enhanced dynamic scans were obtained. The scan delay time was determined using the bolus-tracking technique (CARE Bolus, Siemens Medical Systems; and SmartPrep, GE Healthcare). To obtain time attenuation curves, a small region of interest was placed over the abdominal aorta. Portal venous phase CT scans were automatically initiated 30 seconds after contrast enhancement of the aorta reached the preferred point (100 Hounsfield units), and delayed-phase CT scans were acquired 90 to 110 seconds after the start of the contrast injection. All contrast-enhanced CT images were directly interfaced to our picture archiving and communications system (PACS; Maroview, Marotech, Seoul, Korea).
For MDCT, the CT parameters were as follows: detector collimation, 0.6 to 1.5 mm; gantry rotation time, 0.5 seconds; effective section thickness, 3.0 mm; reconstruction interval, 3.0 mm; 200 reference mA; 120 kVp; and a 512 × 512 matrix size. All CT images were subjected to multi-planar reconstruction of coronal sections for 3-mm section thickness reconstruction.
Analysis of CT images
The following six measurements and analyses of continuous images were independently performed by two gastroenterologist (H.K.J. and G.H.K.) without any information regarding the study subjects. The final data for each subject were obtained by calculating the mean measurement values by the two experts. The angle of His, hiatal hernia, size of the diaphragmatic hiatus, and length of the abdominal esophagus were measured using CT images using methods of previous study [12]. The techniques of the following measurements performed by gastroenterologist were verified and closely monitored by two radiologist (N.K.L. and S.K.).
Angle of His
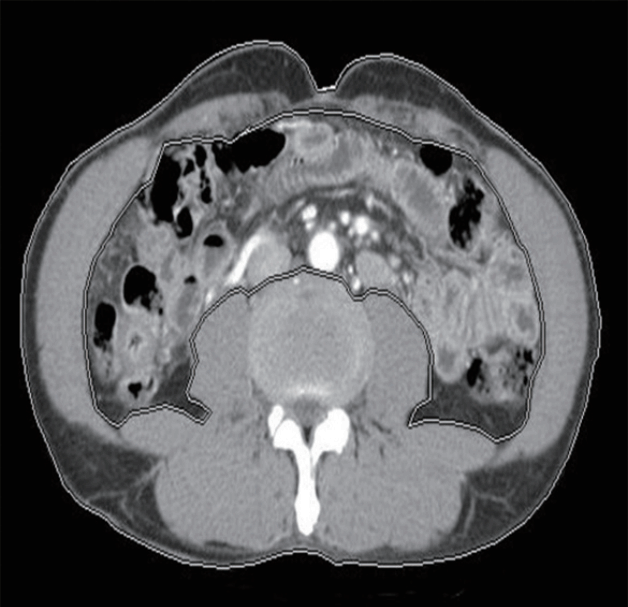
The angle formed by the abdominal esophageal wall and the right wall of the gastric fornix was measured as the angle of His on coronal reformatted images (Fig. 3A).
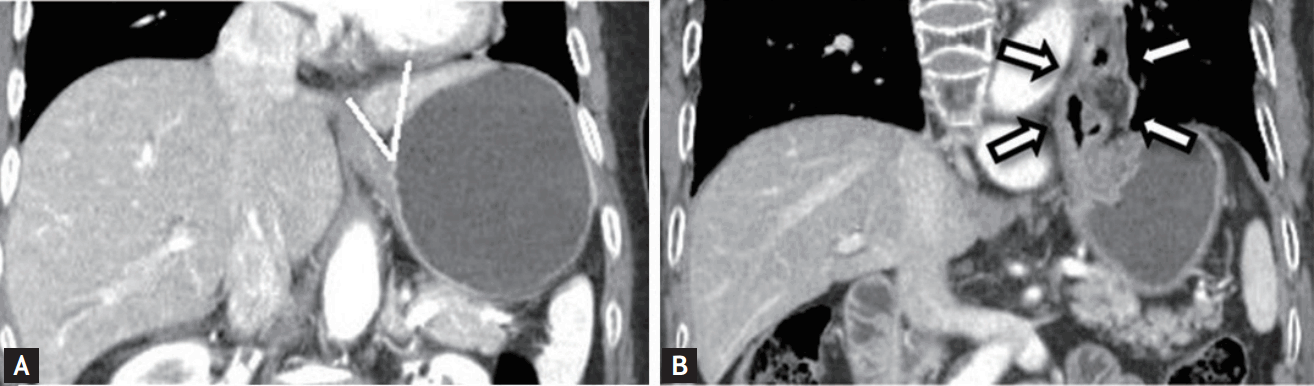
(A) The angle (white line) formed by the abdominal esophageal wall and right side wall of the gastric fornix is measured as the angle of His. (B) A large hiatal hernia (arrows) can be seen on coronal computed tomography. Hiatal hernia was diagnosed when a gastric wall 2-cm or longer was present above the diaphragm.
Hiatal hernia
In the coronal view, a diagnosis of hiatal hernia was made when a 2-cm or longer gastric wall was present above the diaphragm (Fig. 3B).
Size of the diaphragmatic hiatus
The size of the horizontal plane of the esophagus or stomach at the level of the diaphragm was measured to determine the diaphragmatic hiatal size. First, the longitudinal axis of the esophagus or stomach was determined at the level of the diaphragm on coronal reformatted images (Fig. 4A). The horizontal plane perpendicular to the longitudinal axis was determined and the horizontal size of the diaphragmatic hiatus was measured in mm2 (Fig. 4B).
Length of the abdominal esophagus
The length of the esophagus from the diaphragmatic hiatus to the GEJ was measured as the length of the abdominal esophagus. When a hiatal hernia was present, the GEJ was considered to be above the diaphragm. In such cases, the length was determined as a negative value.
Visceral and subcutaneous fat
Abdominal fat areas (cm2) including subcutaneous fat and visceral fat were measured at the level of the first lumbar vertebra (L1) (Fig. 5) [18]. The outer boundary of the abdominal wall was first selected manually as the area of interest using a graph pen. For the visceral fat area, the inner boundary of the abdominal wall muscles and paraspinal muscles were first selected manually as the area of interest using a graph pen. Areas containing fat were automatically selected by applying a CT Hounsfield value threshold (CT attenuation of fat tissue was defined to be between –150 and –50 Hounsfield units) and the surface area of the fat content was measured.
Statistical analysis
Data were expressed as the mean ± SD. A Kruskal-Wallis test was used to assess the statistical significance among the four groups (GEFV grade I to IV) of continuous variables, such as age, body mass index (BMI), angle of His, size of the diaphragmatic hiatus, length of the abdominal esophagus, subcutaneous and visceral fat, and a Mann-Whitney U test was used for comparisons of two groups when Kruskal-Wallis results showed a significant difference among the four groups. The associations between categorical variables, including sex, reflux esophagitis, hiatal hernia, H. pylori infection, and atrophic gastritis according to GEFV grade, were assessed using the chi-square test. A p < 0.05 was considered statistically significant. Statistical calculations were performed with SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Study population
The baseline characteristics of the 138 patients are summarized in Table 1. The total of study population comprised 81 male and 57 female patients, with a mean age of 64.3 years (range, 40 to 84). There were no significant differences in sex and age according to GEFV grade. Thirteen patients (9.4%) showed reflux esophagitis on EGD: 10 had grade A and three had grade B. Most patients with reflux esophagitis had abnormal GEFV (three with normal GEFV vs. 10 with abnormal GEFV). Hiatal hernia was observed in seven patients (5.0%); all had abnormal GEFV. Increased GEFV grade was significantly associated with an increased prevalence of both reflux esophagitis and hiatal hernia (p < 0.001). H. pylori infection was present in 78 patients (56.5%), and open-type atrophic gastritis was observed in 81 patients (58.6%). With the exception of reflux esophagitis and hiatal hernia, the other baseline characteristics including BMI, smoking, alcohol drinking, H. pylori infection and grade of atrophic gastritis, were comparable among the four groups.
Analysis of CT images of the esophagogastric region
Hiatal hernia (≥ 2 cm) was observed in only three patients with GEFV grade IV (Table 2). The mean degree of the angle of His overall was 69.4˚ ± 21.7˚, and the angle of His increased significantly as the GEFV grade increased (65.2˚ ± 19.6˚ in grade I, 66.6˚ ± 19.8˚ in grade II, 76.7˚ ± 11.9˚ in grade III, and 120.0˚ ± 30.3˚ in grade IV, p < 0.001) (Table 2). As a result, patients with normal GEFV had more acute angle of His than did those with abnormal GEFV (65.8˚ ± 19.6˚ vs. 85.1˚ ± 23.7˚, p < 0.001).

Analysis of the esophagogastric junction on computed tomography according to gastroesophageal flap valve grade
The size of the diaphragmatic hiatus, measured by area detector CT, increased as GEFV grade increased (213.0 ± 53.8 mm2 in grade I, 232.6 ± 71.0 mm2 in grade II, 292.3 ± 99.2 mm2 in grade III, and 584.4 ± 268.3 mm2 in grade IV, p < 0.001). As a result, patients with abnormal GEFV showed a significantly wider diaphragmatic hiatus than did those with normal GEFV (348.5 ± 182.2 mm2 vs. 221.4 ± 62.2 mm2 , p < 0.001).
The length of the abdominal esophagus, determined as the distance from the diaphragmatic hiatus to the GEJ, decreased as GEFV grade increased (34.6 ± 5.8 mm in grade I, 32.0 ± 6.5 mm in grade II, 24.6 ± 7.8 mm in grade III, and –22.6 ± 38.2 mm in grade IV, p < 0.001). Patients with abnormal GEFV had a shorter abdominal esophagus than did those with normal GEFV (15.5 ± 25.3 mm vs. 33.5 ± 6.2 mm, p < 0.001).
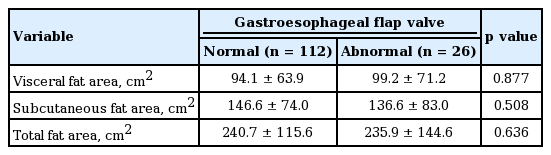
Visceral and subcutaneous fat areas in patients with normal GEFV were 94.1 ± 63.9 and 146.6 ± 74.0 cm2, respectively. Visceral and subcutaneous fat areas in patients with abnormal GEFV were 99.2 ± 71.2 and 136.6 ± 83.0 cm2, respectively. There were no significant differences in visceral and subcutaneous fat areas between patients with normal GEFV and those with abnormal GEFV (p = 0.877 and p = 0.508, respectively) (Table 3).
DISCUSSION
The GEJ is a well-known key defense against gastroesophageal reflux. In addition to its structural and functional properties, anatomy around the GEJ also affects the anti-reflux barrier. Since endoscopic grading of GEFV is a simple and easy method that provides useful information about GERD, it is often used in clinical practice. Based on the hypothesis that anatomical status around the GEJ could be predicted from the GEFV grade, we here compared anatomical factors near the GEJ on abdominal CT according to GEFV grade. As a result, we found a significant correlation between GEFV grade and anatomical changes such as size of diaphragmatic hiatus and length of abdominal esophagus on abdominal CT. However, visceral fat area was not associated with GEFV grade.
The GEFV, formed by gastric cardiac sling musculature, plays an important role as an anti-reflux barrier, together with the diaphragmatic crural fibers and LES [1]. These sling fibers are arranged in a C-shape, with the open side of the C oriented toward the lesser curvature and the closed side located on the greater curvature [1,5]. As fundal volume increases with meal ingestion, the lower end of the esophagus is compressed by direct contact with the gastric wall, augmenting LES pressure [1,3]. This structural relation, referred to as the angle of His in surgical studies, results in a flap valve mechanism [5]. In our previous studies, abnormal GEFV was associated with the presence of GERD, and endoscopic grading of GEFV provided useful information about the status of gastroesophageal reflux [8,9]. In the present study, we also found an increased prevalence of reflux esophagitis in patients with abnormal GEFV.
As noted previously, GEFV is responsible for maintaining an acute angle of His. Grades III and IV flap valves are associated with decreased prominence of the musculomucosal fold near the esophageal inlet. As a result, the angle of His also increases, and there is no longer a subdiaphragmatic segment of the esophagus, namely the abdominal esophagus, to be compressed. Several studies using CT or MRI showed that the angle of His was wider (more obtuse) in GERD patients than in healthy subjects [11,12]. In the present study, the angle of His was wider in patients with abnormal GEFV than it was in those with normal GEFV. In addition, patients with abnormal GEFV showed a shorter abdominal esophagus.
Next, we evaluated the size of the abdominal hiatus according to GEFV grade. Patients with abnormal GEFV had a wider abdominal hiatus than did those with normal GEFV. Hiatal hernia was observed only in three patients with GEFV grade IV. These results are consistent with those of a previous study that showed a positive correlation between GEFV grade and the size of the hiatal defect [19]. On the basis of these findings, surgical or endoscopic treatment for GERD patients can be considered in order to restore the anatomy surrounding the GEJ, including LES pressure [19]. Remarkably, the retroflexed endoscopic appearance of the GEFV is very similar to that of GEFV grade I after a properly performed Nissen fundoplication [10].
Several studies have proposed mechanisms by which obesity causes reflux disease [20-22]. Increased abdominal pressure which relaxes the LES and thus exposes the esophageal mucosa to the gastric content is commonly suggested [21,22]. A case-control study assessing the relation of BMI to esophagitis and hiatal hernia showed that obesity was strongly associated with the combined occurrence of esophagitis and hiatal hernia [23]. In addition, previous studies reported that fat deposits in the visceral and GEJ areas were related to esophageal inflammation and epithelial dysplastic changes [16,24,25]. Based on these findings, esophageal inflammation and metaplasia could be caused by endocrine and paracrine mechanisms as well as by mechanically induced reflux in subjects with increased waist circumference. However, in the present study, we did not find any correlation between GEFV grade and visceral fat area or GEFV grade and BMI. Although we did not directly measure localized fat area around the GEJ, the fat deposit around the GEJ might be more associated with the GEFV grade than might the total visceral fat deposit. Further studies measuring segmental visceral fat deposition will be needed to investigate these findings.
This study has several limitations. First, we used a retrospective design to analyze abdominal CT findings to assess anatomical factors according to GEFV grade. Because GERD patients and healthy individuals do not undergo abdominal CT routinely, we included patients with EGC who underwent both abdominal CT and EGD. However, to minimize the effect of EGC on the anatomy related to anti-reflux barrier, we excluded patients with EGC at the proximal stomach. In addition, due to the limitation of a retrospective design study, we could not figure out the reflux-related symptoms from enrolled patients, and could not evaluate the correlation between anatomical factors and symptoms.
Second, the position of patients and methods for distending the stomach were different between EGD and abdominal CT. EGD was performed while patients were in the left lateral decubitus position, and air inflation was used to distend the stomach. On the other hand, abdominal CT was performed while patients were in the supine position, and water was used to distend the stomach. These differences might have influenced our results. Third, the number of patients with grade IV GEFV was small compared to the number of patients with other GEFV grades. Despite these limitations, our study is meaningful in that we analyzed the association between GEFV grade and anatomical changes around the GEJ, and our results support the clinical importance of GEFV. Further prospective studies including patients with GERD and healthy controls are needed to validate our results for the associations between GEFV and abdominal CT findings.
In conclusion, our study revealed that endoscopic GEFV grading is well correlated with anatomical changes around the GEJ on abdominal CT: the angle of His, the size of the diaphragmatic hiatus, and the length of the abdominal esophagus. Therefore, endoscopic GEFV grading can provide useful information about the anti-reflux barrier of GEJ.
KEY MESSAGE
1. Endoscopic gastroesophageal flap valve (GEFV) grading is well correlated with anatomical changes around the gastroesophageal junction (GEJ) on abdominal computed tomography including the angle of His, the size of the diaphragmatic hiatus and the length of the abdominal esophagus.
2. Endoscopic GEFV grading can provide useful information about the anti-ref lux barrier of GEJ.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family Affairs, Republic of Korea (0920050) and a clinical research grant from the Pusan National University Hospital 2015.