Reference intervals of thyroid hormones during pregnancy in Korea, an iodine-replete area
Article information
Abstract
Background/Aims
Maternal thyroid dysfunction has been associated with adverse pregnancy outcomes. The purpose of our study was to establish trimester-specific reference intervals for thyroid hormones in pregnant women in Korea, where iodine intake is more than adequate and to examine pregnancy and perinatal outcomes in their offspring.
Methods
Among 459 healthy pregnant women who were screened, we enrolled 417 subjects who had negative results for thyroid autoantibodies. Serum thyroid stimulating hormone (TSH) and free thyroxine were measured using an immunoradiometric assay. Urine iodine concentration was measured using inductively coupled plasma-mass spectrometry in 275 women. Reference ranges of thyroid hormones were determined according to the guidelines of the National Academy of Clinical Biochemistry. Pregnancy and perinatal outcomes were compared according to maternal thyroid function.
Results
The reference ranges of serum TSH were 0.03 to 4.24 mIU/L in the first trimester, 0.13 to 4.84 mIU/L in the second trimester, and 0.30 to 5.57 mIU/L in the third trimester. Pregnancy and perinatal outcomes did not vary in mothers with subtle changes in thyroid function.
Conclusions
Trimester-specific thyroid hormone reference intervals in Korean pregnant women differ from those of other countries with different iodine nutrition status and ethnicity. The establishment of population-based, reliable trimester-specific reference intervals is critical for the interpretation of thyroid function in pregnant women to avoid unnecessary tests and treatments.
INTRODUCTION
Over the past few decades, the association between maternal thyroid dysfunction, thyroid antibodies and adverse effects on pregnancy has been studied. Overt thyroid dysfunction is related with adverse obstetric outcomes and needs to be treated in pregnant women [1,2]. However, subclinical hypothyroidism during pregnancy has insufficient evidence for risks of obstetric problems [3,4] and the preventive effects of levothyroxine treatment [5,6]. Moreover, a recent large prospective cohort study suggested potential harmful effects of levothyroxine therapy on the child neurodevelopmental outcomes in pregnant women with subclinical hypothyroidism [7].
As the diagnosis of subclinical thyroid disease depends upon thyroid function test due to the absence of clinical manifestations, determination of trimester-specific reference intervals for thyroid hormones and thyroid stimulating hormone (TSH) is crucial to avoid over- or under-diagnosis of subclinical hypothyroidism in pregnant women [8]. Thyroid function reference ranges vary among different populations, which might be explained by variation in ethnicity, iodine intake, body size as well as assay methodology [8].
The National Academy of Clinical Biochemistry (NACB) recommends that reference intervals for serum TSH should be established using specimens from normal euthyroid subjects excluding individuals having specific features such as goiter and known thyroid disease [9]. When trimester-specific reference values for TSH are not available according to region and iodine intake, American Thyroid Association (ATA) management guidelines for thyroid disease during pregnancy recommend the use of their TSH reference values: 0.1 to 2.5 mIU/L in the first, 0.2 to 3.0 mIU/L in the second, and 0.3 to 3.0 mIU/L in the third trimester [10].
World Health Organization (WHO) recommended epidemiologic criteria for assessing iodine nutrition based on the urine iodine concentration (UIC) in pregnant women [11]. A median UIC of 150 to 249 µg/L is considered as an adequate intake of iodine. A UIC between 250 and 499 µg/L is more-than-adequate, and 500 µg/L or more is defined as excessive iodine intake. We reported the median UIC of 427.3 µg/L in Korean pregnant women, which is more-than-adequate according to WHO criteria [12].
Recently, the TSH and free thyroxine (fT4) reference intervals are reported in 465 Korean pregnant women using electrochemiluminescence immunoassay (Roche Diagnostics, Mannheim, Germany) [13]. However, the previous study has limitations in that they did not investigate iodine status and pregnancy outcomes in their population [13].
Here, we aimed to establish trimester-specific reference intervals for TSH and fT4 using immunoradiometric assay (Beckman Coulter, Brea, CA, USA), complying with the NACB guidelines. In addition, we examined pregnancy and perinatal outcomes according to the new TSH reference intervals.
METHODS
Study population
From April 2012 to March 2014, healthy pregnant women who visited Samsung Medical Center for routine antenatal checkups were consecutively and prospectively enrolled. Exclusion criteria included women with a visible or palpable diffuse or nodular goiter, a history of thyroid disease or medication usage, positive for thyroid autoantibodies and multifetal gestation. Among a total of 459 women who were screened for thyroid autoantibodies, 42 (9.2%) were excluded for positive thyroid autoantibodies; thus, a total of 417 subjects were enrolled in this study (Table 1).
Pregnancy and perinatal outcomes
Pregnancy and perinatal outcomes were available in 371 pairs of mothers and their singleton babies after exclusion of 46 pregnant women who gave birth at outside hospitals. Gestational hypertension was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg during pregnancy. Severe preeclampsia was defined as women with hypertension and ≥ 1 of following conditions; blood pressure ≥ 160/110 mmHg, serum creatinine > 1.1 mg/dL, thrombocytopenia (< 100,000 platelets/µL), serum transaminase concentration more than twice an upper normal limit, persistent headache or scotomata [14]. Major malformations in infants included anomalies in principal organs, an identifiable syndrome and aneuploidy [15]. Neonatal TSH was checked using a capillary heel stick blood sampling between 3 and 7 days after birth for a routine newborn screening. Informed consent was obtained from all the participants and the Institutional Review Board of Samsung Medical Center approved this study.
Laboratory tests
Maternal blood samples were obtained at the time of enrollment and stored at –80°C. Serum TSH, fT4 was measured using an immunoradiometric assay. The intra-and interassay coefficients of variation were ≤ 3.7% and 8.6% for TSH, and ≤ 10.29% and 7.58% for fT4, respectively. Anti-thyroid peroxidase (anti-TPO) antibody was determined using a radioimmunoassay kit (BRAHMS AG, Hennigsdorf, Germany) and anti-thyroglobulin (anti-Tg) antibody was measured using an anti-Tg RIA kit (BRAHMS AG). Anti-TPO antibody and anti-Tg antibody less than 60 U/mL were considered negative according to the manufacturer’s instructions. All samples were measured in triplicate.
Among 417 women, urine samples were obtained from 275 subjects at the time of blood sampling. Urinary iodine was measured by inductively coupled plasma-mass spectrometry using an Agilent 7500 series instrument (Agilent Technologies Inc., Tokyo, Japan) as described in a previous study [16].
Statistical analysis
SPSS version 18 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Descriptive characteristics were tabulated for baseline characteristics using one-way analysis of variance for parametric statistics and the Kruskall-Wallis test for non-parametric statistics, if appropriate. Percentile values for TSH and fT4 were presented with 2.5th, 5th, 25th, 50th, 75th, 95th, and 97.5th percentiles. Reference ranges for TSH and fT4 in each trimester were defined as the range between 2.5th and 97.5th percentiles according to NACB recommendations. Women with TSH above the 97.5th percentile but normal fT4 based on reference values determined from our population were regarded as having subclinical hypothyroidism.
RESULTS
Baseline characteristics
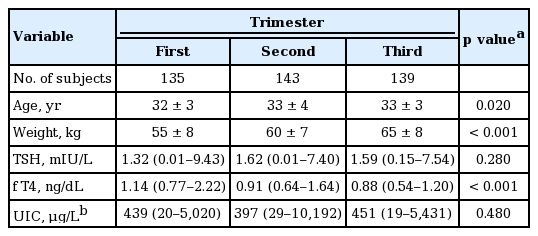
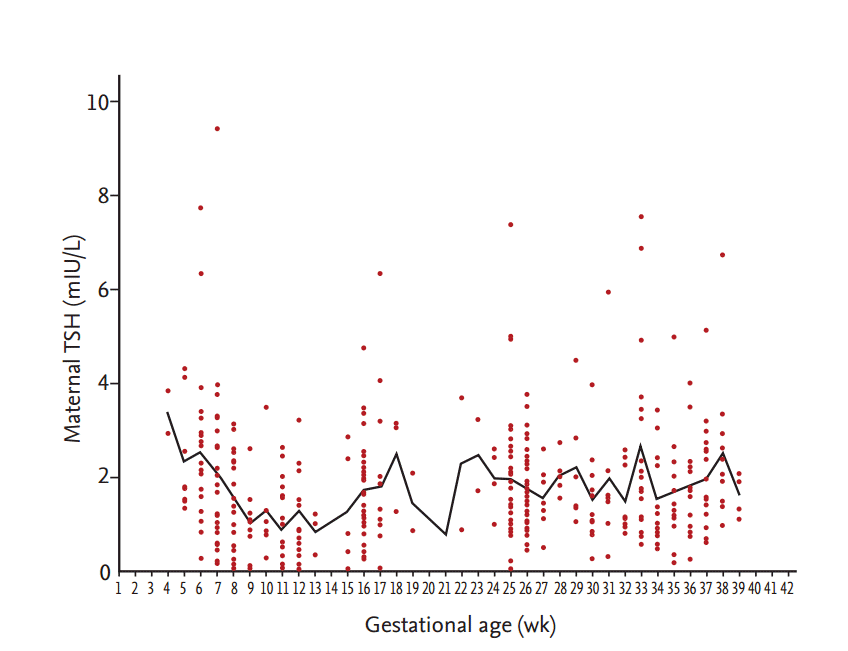
A total of 417 healthy pregnant women were eligible for analysis; 135 women in the first trimester, 143 in the second trimester and 139 in the third trimester. The baseline demographics are presented in Table 2. The mean age at study enrollment was 33 years. Mean weight was 60 kg and as expected, maternal body weight increased with advanced gestational age (GA; p < 0.001). Median TSH was 1.32, 1.62, and 1.59 mIU/L in each trimester (Fig. 1). Median fT4 was 1.14, 0.91, and 0.88 ng/dL, respectively (p < 0.001). Median UIC of all subjects was 429 µg/L and no difference was observed among each trimester.
Reference intervals of TSH and fT4 in each trimester
Percentile values of TSH and fT4 are presented as 2.5th, 5th, 25th, 50th, 75th, 95th, and 97.5th percentiles in each trimester (Table 3). Reference intervals of TSH were defined as the range between the 2.5th and 97.5th percentiles; TSH between 0.03 to 4.24 mIU/L in the first trimester, 0.13 to 4.84 mIU/L in the second trimester, and 0.30 to 5.57 mIU/L in the third trimester. For fT4, reference intervals were between 0.84 to 1.43 ng/dL in the first trimester, 0.68 to 1.21 ng/dL in the second trimester, and 0.67 to 1.13 ng/dL in the third trimester.
Using the ATA guidelines for pregnant women in our population, 34 women (25.2%) in the first trimester, 18 (12.6%) in the second trimester, and 18 (12.9%) in the third trimester met criteria for subclinical hypothyroidism (Table 3).
Pregnancy and perinatal outcomes according to maternal thyroid function
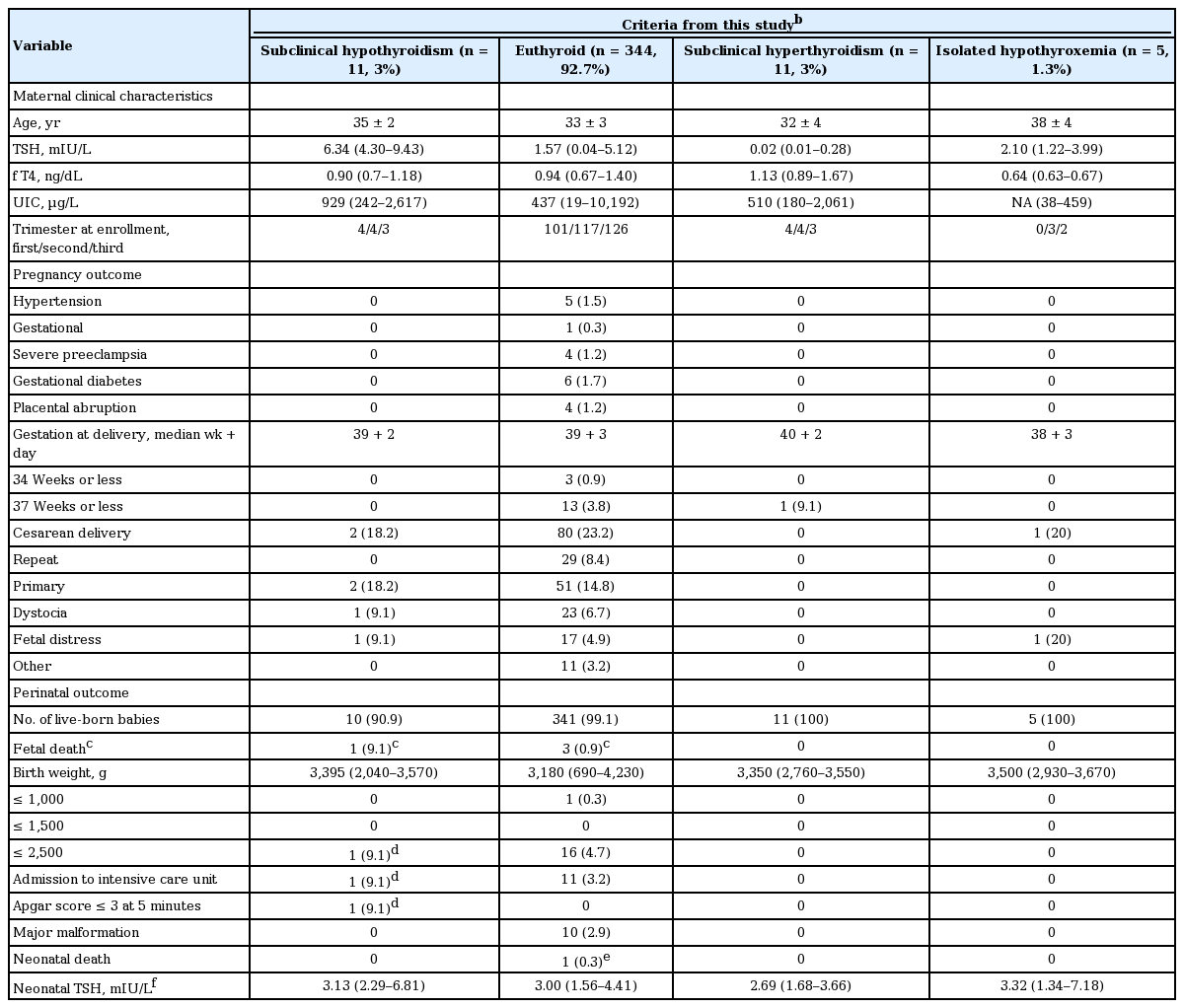
Pregnancy and perinatal outcomes for 371 pairs of mothers and babies (including fetal death) were presented in Table 4. Maternal thyroid function was determined by criteria established in our population. Among 371 mothers, 344 mothers (92.7%) showed euthyroid status, 11 (3%) with subclinical hypothyroidism, 11 (3%) with subclinical hyperthyroidism, and five (1.3%) with isolated hypothyroxemia. There was no difference in pregnancy and perinatal outcomes among mothers with different thyroid functional status. The median neonatal TSH was higher in babies from mothers with subclinical hypothyroidism and isolated hypothyroxemia than those from mothers with euthyroid and subclinical hyperthyroidism, but the difference was not significant. A male baby from subclinical hypothyroid mother entered intensive care unit for fetal asphyxia. He was born with small for GA (2,040 g) at GA 37 + 2 (week + day). His mother was elderly pregnant women (42-year-old) without any pregnant complication. Her serum TSH was 9.43 mIU/L at GA 7 + 2, but spontaneously decreased to 2.11 mIU/L at GA 11 + 2 and 2.80 mIU/L at 21 + 4 without medication.
DISCUSSION
This prospective, observational study suggested that trimester-specific reference intervals of TSH in pregnant Korean women were different from those in other countries with different levels of iodine intake. We strictly followed the NACB guidelines to set up normal reference intervals of TSH among pregnant Korean women in this study. The normal reference intervals obtained from this study (0.03 to 4.24 mIU/L for the first, 0.13 to 4.84 mIU/L for the second, and 0.30 to 5.57 mIU/L for the third trimester) have a wider range than those recommended by the ATA guidelines. In addition, we evaluated neonatal outcomes according to maternal thyroid function.
Our data showed that among pregnant women without known thyroid disease in Korea, 6.1% of women tested positive for TPO antibody and 4.4% of women tested positive for Tg antibody. These prevalence rates are lower than those of previous studies that reported prevalence rates for TPO antibody of 10.4% to 19.8% and Tg antibody of 5.5% to 15.7% [17,18]. This may be explained by the existence of ethnic differences and the enrollment of women without apparent goiters and without a history of thyroid disease in this study.
After exclusion of women positive for thyroid autoantibodies, the upper reference limit of trimester-specific TSH reference values established in this study was higher than those of other studies from Switzerland [17], the United States [19], and Spain [20], which reported upper TSH limits of 2.83 to 2.90 mIU/L in the first, 2.78 to 2.99 mIU/L in the second, and 2.64 to 3.56 mIU/L in the third trimester [17,19,20]. On the other hand, the reference intervals in this study were comparable to those reported in India [21] and China [22]. Owing to substantial variation of TSH reference intervals among different populations, each institution is recommended to set their own trimester-, assay-specific reference ranges for pregnant women [8,10].
Recently, Moon et al. [13] reported reference intervals (2.5th to 97.5th percentile) for TSH and fT4 in 465 Korean pregnant women: for TSH 0.01 to 4.10 mIU/L (first trimester), 0.01 to 4.26 mIU/L (second trimester), and 0.15 to 4.57 mIU/L (third trimester); and for fT4, 0.83 to 1.65 ng/dL (first trimester), 0.71 to 1.22 ng/dL (second trimester), and 0.65 to 1.13 ng/dL (third trimester). We confirmed wider ranges of TSH and fT4 reference intervals in Korean pregnant women using different assay (Beckman Coulter) and provided additional information on iodine status which is one of the factors affecting maternal thyroid function.
Korea is an iodine-replete country and the median UIC of 429 µg/L in healthy pregnant women who had no evidence of thyroid disease, exceeding those of pregnant women living in nearby Asian countries [18,23]. Although adequate iodine intake is essential for normal fetal brain development and the synthesis of maternal thyroid hormones, excessive iodine status is related to maternal subclinical hypothyroidism [18]. Sang et al. [18] reported the higher prevalence of subclinical hypothyroidism (20%) in pregnant women with excessive iodine intake compared to those with adequate iodine intake (2.3%), but the rates of overt hypothyroidism were similar (0.5% vs. 0%).
According to the reference ranges provided by current ATA guidelines, the prevalence of subclinical hypothyroidism was 25.2% in the first trimester, 12.6% in the second trimester, and 12.9% in the third trimester in the present study. That is, about one quarter of pregnant women would be possible candidates for levothyroxine treatment in the first trimester. Li et al. [22] drew similar conclusions in Chinese pregnant women of a high prevalence of subclinical hypothyroidism (27.8%) based on a fixed upper limit of 2.5 mIU/L in early pregnancy. Interestingly, Korevaar et al. [7] reported that both low and high maternal fT4 levels during pregnancy resulted in lower child IQ (a median of 6.0 years of age) and lower gray matter and cortex volume, warning the potential adverse effects of preventive levothyroxine therapy in subclinical hypothyroid mothers.
Maternal subclinical hypothyroidism has been described as a factor for pregnancy loss, premature delivery, low birth weight (LBW) and hypertensive disorder, but results are inconclusive [2,3,8]. A recent meta-analysis concluded that subclinical hypothyroidism and isolated hypothyroxemia did not increase the risk of preterm birth [2]. A study using the National Birth Registration (NBR) data reported that a prevalence of preterm birth (< 37 weeks gestation) between 3.4% to 5.9% and LBW (< 2,500 g) between 3.2% to 5.2% in Korea (2004 to 2008) [24], and our results are comparable with the prevalence from NBR database. One baby with LBW and low Apgar score who admitted to intensive care unit was born from a 42-year-old mother who showed subclinical hypothyroidism (TSH of 9.43 mIU/L) at GA 7 + 2. In general, serum TSH levels decrease from the 8th weeks throughout the first half of pregnancy [22], and her TSH was normalized without any intervention. Thus, it is expected that the perinatal outcome might be derived from the elderly gravida rather than maternal thyroid dysfunction. We reported no difference in pregnancy and perinatal outcomes between subclinical hypothyroid and euthyroid mothers and their babies using TSH reference intervals determined in our population, although the number of women with subtle changes of thyroid function was too small to compare these outcomes. Excessive iodine intake is related to the subclinical hypothyroidism, but data is limited which subclinical thyroid diseases in mothers affect the short and long-term outcomes in offspring, especially in iodine-replete area. Therefore, the relationship between minor variation in maternal thyroid function using population-based reference intervals and offspring’s outcomes such as intellectual, behavioral development and thyroid function needs to be validated in larger cohort studies.
There are some limitations to this study. First, this study only used one kit for serum TSH and fT4 measurements and was not tested using different kits. Thus, these reference intervals need to be used with caution when different kits are used. Fortunately, the measurement of serum TSH is better internationally standardized compared to other blood tests for thyroid function including fT4. Second, this study was conducted at a tertiary referral hospital and not in a primary care hospital setting. However, the Obstetric Department of our institution functions not only as a tertiary referral hospital, but also as a primary care center for pregnant women in this area. This is because the hospital is located in a populated area, where 44% of entire population resides, but only a few delivery hospitals are nearby due to poor reimbursement for childbirth from the National Health Insurance System. Considering that the percentages of preterm birth, LBW and congenital malformation are similar to the prevalence reported from NBR database and multicenter data (birth defect of 2.7% in this study vs. 1.8% in multicenter data) [25], we are confident that the participants in this study are not deviated for high-risk pregnant women, but they seem not to be representative of average pregnant women in Korea due to limited number of subjects. Third, maternal TSH concentrations in each trimester were not measured serially in the same pregnant women. However, we think this does not affect the study aim which is to set the reference intervals of serum TSH level in each trimester during pregnancy in Korea. Forth, UIC was measured in limited number of subjects and fetal outcome was not identified in babies born at outside hospital. Fifth, we did not study the long-term neuropsychological development of children born from mothers with different levels of serum TSH. We identified no adverse effect on perinatal outcome of subclinical hypothyroid mothers, but it does not guarantee the long-term safety for their offspring; thus, population-based studies are warranted to clarify this issue.
In conclusion, this study provides trimester-specific reference intervals of thyroid hormones in pregnant Korean women that differed from those of pregnant women in other countries with different ethnicity and iodine nutrition. These findings will reduce the number of pregnant women who may be classified as having subclinical hypothyroidism and are subjected to unnecessary levothyroxine treatment. In addition, education about adequate iodine intake during pregnancy to prevent both inadequate and excessive iodine intake must be emphasized. Further investigations on the association between thyroid hormones and clinical outcomes are needed.
KEY MESSAGE
1. Korean pregnant women have wider ranges of thyroid hormone reference intervals and pregnancy outcomes did not vary in mothers with subtle changes in thyroid function.
2. These findings will reduce the number of pregnant women who may be classified as having subclinical hypothyroidism and are subjected to unnecessary levothyroxine treatment.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by a Samsung Medical Center grant (#PHO 1125465).