Predictors of acute myocarditis in complicated scrub typhus: an endemic province in the Republic of Korea
Article information
Abstract
Background/Aims
Scrub typhus is known as a self-limited infectious disease. Cardiac complication is uncommon and usually not life-threatening. Until now, few cases of fulminant myocarditis by scrub typhus have been reported. So, we investigated incidence and predictors of acute myocarditis in severe scrub typhus.
Methods
We retrospectively reviewed 89 patients among 91 scrub typhus confirmed patients who examined an echocardiogram and cardiac biomarkers from 2005 to 2015 in the intensive care unit at our hospital. We excluded two patients who didn’t have electrocardiography. Patients were divided into two groups and compared between scrub typhus with (n = 13) and without (n = 76) acute myocarditis.
Results
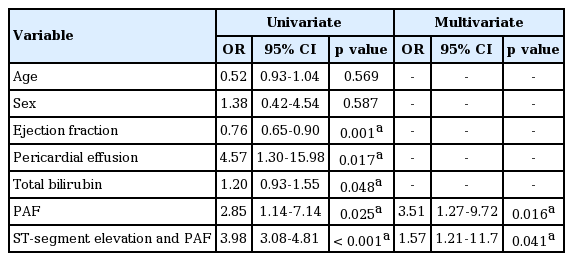
Age, sex, and underlying diseases were similar between the groups. The existence of eschar and duration of general ache and fever were similar between the groups. However, patients with acute myocarditis had more elevated total bilirubin, high incidence of ST elevations and paroxysmal atrial fibrillation (PAF) than those without acute myocarditis. Receiver operating characteristic analysis showed that the PAF was a predictor of myocarditis with a sensitivity of 70% and specificity of 84%. Predictive power of combination of ST-segment elevation and PAF was significantly associated with myocarditis in the multivariate analysis (odds ratio, 1.57; 95% confidence interval [CI], 1.21 to 11.7; p = 0.041) and area under the curve was 0.947 (95% CI, 0.878 to 0.983; p < 0.001).
Conclusions
Acute myocarditis with scrub typhus may be more common than previously reported. Patients with high bilirubin and PAF are at increased risk of acute myocarditis with scrub typhus. These patients warrant closer follow-up and echocardiogram would be needed.
INTRODUCTION
Scrub typhus is a zoonotic disease caused by Orientia tsutsugamushi confined to East Asia and the Western Pacific islands [1]. It is characterized by fever, headache, mental confusion, rash, and eschar. The majority of patients without complications can recover with an early diagnosis and proper management with doxycycline or tetracycline. Despite antibiotic treatment, scrub typhus with severe complications rarely progresses to a fatal disease including sepsis and myocarditis. In previous reports [2-4], the mortality rate of scrub typhus ranges from 16.7% to 30% in Southeast Asia. Severe complications can be manifested by acute respiratory distress syndrome, encephalitis, interstitial pneumonia, acute renal failure, acute hepatic failure, and acute myocarditis [5]. In the case of fulminant scrub typhus myocarditis, its complications can lead to cardiogenic shock and cardiac arrest. But, it has rarely been reported. Therefore, predictors for associated myocarditis is needed to provide a timely and appropriate diagnosis and to reduce the mortality rate of complicated scrub typhus; therefore, we did this study to evaluate predictors of acute myocarditis in patients with scrub typhus admitted to a 3rd referral center in the intensive care unit (ICU), retrospectively.
METHODS
Study patients
We retrospectively analyzed data from 91 patients who were consecutively admitted to the ICU, Division of Infection, at Eulji University Hospital, a 3rd referral center, and diagnosed with severe and complicated scrub typhus between October 2005 and May 2015. The diagnosis of scrub typhus in patients was based on the World Health Organization’s criteria. When an immunofluorescent antibody assay (IFA) titer against O. tsutsugamushi increased more than four times or an indirect IFA IgM titer against O. tsutsugamushi was ≥ 1:80, scrub typhus was confirmed. The following data were collected from an electronic data system and medical records.
Diagnosis of scrub typhus and complicated myocarditis
Patients with severe complicated scrub typhus were first admitted to the emergency room and transferred to the ICU according to the physician’s decision based on clinical presentation and objective data. We divided all the patients into the following two groups: scrub typhus with (n = 13) and without (n = 76) myocarditis groups. The diagnosis of myocarditis in patients was based on the diagnostic criteria for clinically suspected myocarditis presented by European Society of Cardiology [6]. We considered myocarditis clinically if patients had one or more clinical presentations and one or more diagnostic criteria from different categories. Clinical presentations were (1) acute chest pain, (2) new-onset dyspnea, (3) worsening of dyspnea, (4) palpitation, and/or unexplained arrhythmia symptoms, and/or syncope, and/or acute chest pain during exercise, and (5) unexplained cardiogenic shock. Diagnostic criteria were (1) abnormal electrocardiography (ECG) features, (2) elevation of cardiac biomarkers, and (3) functional and structural abnormalities on cardiac imaging including echocardiogram and coronary angiogram to confirm the etiology of ischemic origin.
Electrocardiography
Standard 12-lead ECGs and ICU rhythm strip were recorded at 25 mm/sec speed and 10 mm/mV upon admission. The ECG recordings were reviewed by two independent investigators blinded to clinical and echocardiogram data. ECGs were evaluated for atrial fibrillation (AF), AV-block, bundle-branch block, waves, and ST-abnormalities. The presence of RS in V1 lead, R > 40 ms in V1 lead, and QR or low voltage (R wave < 5 mm) were considered as Q wave equivalent. ST-abnormalities were defined as significant ST-segment elevation and/or significant ST-segment depression and/or T-wave inversion [7]. ST elevation or depression, respectively, exceeding 1 mm measured 60 ms after the J point was considered significant. An AF episode is defined as irregular supraventricular tachyarrhythmia that is documented by ECG monitoring and has duration of at least 30 seconds, or if less than 30 seconds, is present continuously throughout the ICU rhythm monitoring tracing. Especially, paroxysmal atrial fibrillation (PAF) that terminates spontaneously or with intervention within 7 days of onset and episodes may recur with variable frequency in the ICU telemetry [8,9].
Echocardiography
Standard echocardiography was performed by experienced physicians or sonographers using portable echocardiographic machine (GE VIVID S3 apparatus, GE Medical systems, Milwaukee, WI, USA) with a 2.5-MHz phased array transducer in ICU. Left ventricular (LV) volume, LV mass index, LV ejection fraction, and status of inferior vena cava were measured by the American Society of Echocardiography guidelines [10].
Coronary angiography
An invasive coronary angiogram was performed subsequently for evaluation of ischemic cardiomyopathy in all patients with scrub typhus myocarditis. The conventional Judkins’ technique was used with at least four views of the left and two views of the right coronary artery. Lesions were examined in orthogonal views, and severity of luminal stenosis was determined by the consensus of two experienced cardiologists who performed quantitative assessment of stenosis severity using standard techniques with an automated edge-detection algorithm (CASS V, Pie Medical Imaging, Maastricht, the Netherlands).
Statistical analysis
Continuous variables were compared with the two-tailed Student t test. Categorical data were analyzed with the chi-square test. Factors associated with mechanical ventilation were analyzed with binary linear regression models. Univariate binary logistic regression analysis was done. Then, multivariate analysis was done with the significant factors in the univariate analysis. Results were reported as the mean ± standard deviation or number (%). Data were collected in Microsoft EXCEL (Microsoft Excel 2007, Microsoft Corp., Seattle, WA, USA). Receiver operating characteristic (ROC) analyses were done to determine the diagnostic precision of our index for myocarditis. Statistical analyses were done with SPSS version 15.0 (SPSS Inc., Chicago, IL, USA) and MedCalc version 12.3 (MedCalc software, Ostend, Belgium). A p value of < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
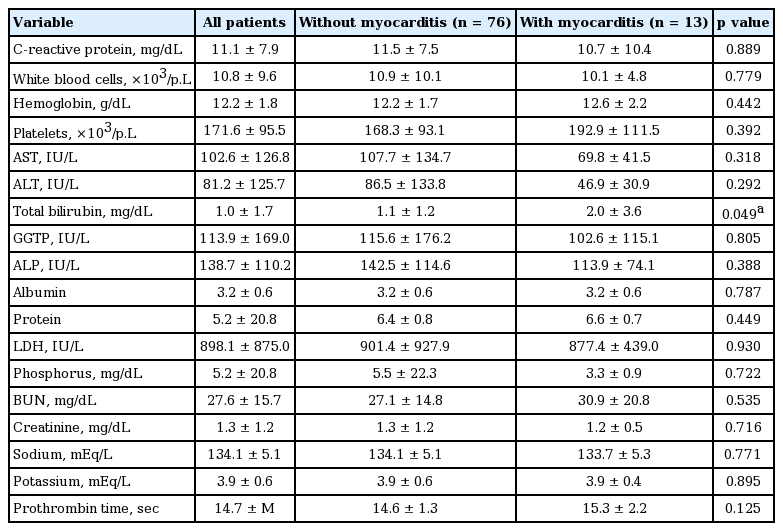
The proportion of scrub typhus myocarditis was 14% in this study. The mean age of all patients was 69; the proportion of males was 39.3%. The mortality of scrub typhus myocarditis was 15.5% in the ICU. Table 1 shows that age, gender, proportion of hypertension, diabetes, and coronary artery disease were similar between the two groups. For clinical features, the proportion of skin lesion like rash, eschars, and duration of general ache and fever were similar between the two groups. Table 2 shows that the laboratory findings including liver enzymes and except for total bilirubin were not significantly different between the two groups. Scrub typhus with myocarditis had significantly more elevated total bilirubin than scrub typhus without acute myocarditis (2.0 ± 3.6 vs. 1.1 ± 1.2, p = 0.049).
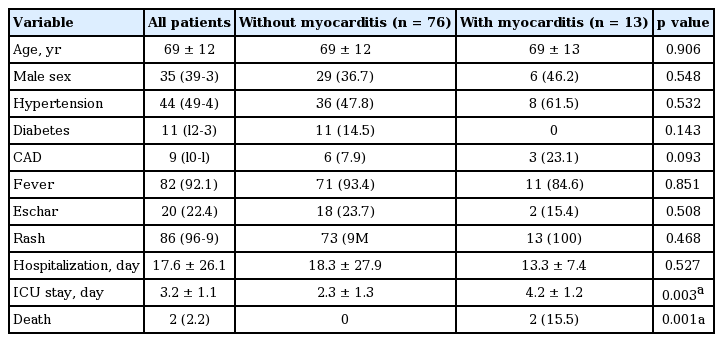
Baseline clinical characteristics of patients with scrub typhus with and without myocarditis admitted to the ICU
ECG, echocardiogram, and coronary angiogram
In the patients with scrub typhus myocarditis, ST-segment abnormalities were detected most frequently (46.1%) than Q-wave (15.5%) in the ECG on ICU admission (Table 3). ST-segment abnormalities and Q wave were not associated with a clinical presentation such as severe chest pain and acute onset dyspnea. Diffuse ST-segment elevation has been considered as the most frequent feature among the ECG parameters associated with acute myocarditis, which may be explained by direct sign and phenomenon of myocardial injury. In addition, PAF was presented more frequently in the patients with scrub typhus myocarditis (38.5%) than without myocarditis (21.0%, p = 0.002).
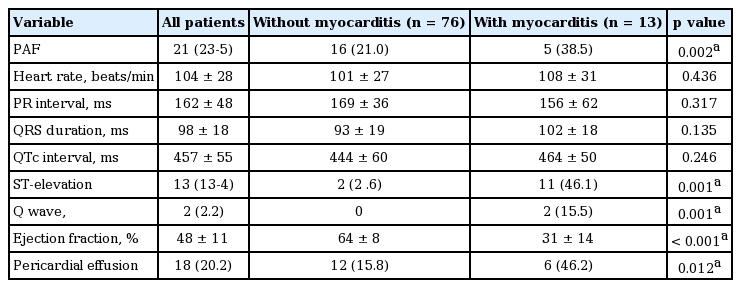
Electrocardiography and echocardiogram of the patients with scrub typhus with and without myocarditis admitted to the intensive care unit
Scrub typhus with acute myocarditis had lower LV ejection fraction (64% ± 8% vs. 31% ± 14%, p < 0.001) but, recovered to normal LV function afterward. (Supplementary Table 1). One patient among those with acute myocarditis had normal LV ejection fraction with diffuse LV wall swelling by echocardiogram. Six patients among those with acute myocarditis had minimal to small amount of pericardial effusion. Most of the patients had normal or insignificant lesions in coronary arteries except one patient with percutaneous coronary intervention.
Total bilirubin and PAF in myocarditis
Patients with scrub typhus myocarditis had significantly more PAF than scrub typhus without acute myocarditis. ROC analysis showed that PAF was predictive for myocarditis with a sensitivity of 70% and specificity of 84% and an area under the curve (AUC) of 0.766 (p < 0.001) shown in Fig. 1. In the multiple logistic regression, PAF shows a significant correlation with scrub typhus myocarditis (odd ratio [OR], 3.51; 95% confidence interval [CI], 1.27 to 9.72; p = 0.016) (Table 4). The patients with PAF (n = 21, 24%) had older age, low albumin, and low protein level (Supplementary Tables 2 and 3). ECGs of patients with PAF showed higher heart rate and high incidence of ST elevatation. (Supplementary Table 4). The predictive power of combination of ST-segment elevation and PAF was significantly associated with myocarditis in the univariate analysis (OR, 3.98; 95% CI, 3.08 to 4.81; p < 0.001) and multivariate analysis (OR, 1.57; 95% CI, 1.21 to 11.7; p = 0.041). In the ROC analysis, AUC was 0.947 (95% CI, 0.878 to 0.983; p < 0.001) with Youden index 0.89.

Receiver operating characteristic curves for paroxysmal atrial fibrillation in patients with scrub typhus myocarditis.
DISCUSSION
Until now, scrub typhus myocarditis has been not commonly reported in a clinical infectious state. However, here, we present the clinical feature of acute myocarditis caused by scrub typhus which may present with fatal complications. In our study, PAF was significantly associated with scrub typhus myocarditis.
The pathophysiology of scrub typhus is disseminated vasculitis with subsequent vascular damage that involves multiple organs. The organism multiplies at the site of inoculation and progresses to necrosis and evolves into an eschar with regional lymphadenopathy [11]. Complicated scrub typhus infection includes pneumonia, acute respiratory distress syndrome, encephalitis, hepatitis, disseminated intravascular coagulopathy, acute kidney injury, acute pancreatitis, subacute thyroiditis, and acute myocarditis [12].
Acute myocarditis associated with heart failure results in a broad range of elevated liver enzymes. The most common finding is a mild elevation of bilirubin with increases in other cholestatic markers such as alkaline phosphatase and γ-glutamyl transpeptidase being milder and less frequent, up to 10% to 20%, as well as alanine aminotransferase (12% to 33%) [13]. The etiology for heart failure to liver damage has been considered to be derived from two hemodynamic alterations: decreased hepatic blood flow originating from low cardiac output and increased hepatic venous pressure with subsequent atrophy of liver cells and edema of the peripheral area, both leading to hepatocellular hypoxia [14]. In a previous study, elevated total bilirubin such as liver enzymes was significantly associated with clinical and biochemical signs of marked systemic congestion and elevated right-sided filling pressure, including peripheral edema, ascites, and tricuspid regurgitation. In acute decompensated heart failure, the marked increase in the vena cava and centrilobular pressure is transmitted back to the liver sinusoids [14,15]. Total bilirubin such as liver enzymes also may be interpreted as a surrogate marker showing improvement in heart function and in liver congestion which might lead to reopening of the biliary tract and to reductions in liver enzyme. As previously reported, we showed that elevated liver enzymes including bilirubin, alkaline phosphatase, and alanine transferase were more elevated in scrub typhus. Especially, among them, total bilirubin was significantly associated with the presentation of scrub typhus myocarditis (Table 2).
Cytokines produced by activated cells, usually monocytes and macrophages, in response to inflammatory stimuli were also involved in the pathogenesis of AF. Previous study has suggested that cardiac involvement by inflammation associated with atrial myocarditis with subsequent aggravating electrical and structural atrial changes, resulting in the initiation and maintenance of AF [16]. They are paramount in activating the inflammatory cascade and in the production of acute-phase proteins in the PAF [16,17]. Therefore, the mechanisms of AF also can be explained by initiating multiple random propagating wavelets, focal electrical discharges, and substrate localized reentrant activity with fibrillatory conduction. It also suggested the concept that the myocarditis developed PAF as a trigger underling an anatomic or electrophysiologic substrate capable of both initiation and perpetuation of AF [8].
In our study, PAF was significantly associated with scrub typhus myocarditis (Table 3). ST abnormalities can be found in most patients with biopsy proven viral myocarditis at initial presentation [7]. ECG findings are related to the amount and area of damage as indicated by cardiac magnetic resonance, which confirms the important clinical role of the ECG for the diagnosis of viral myocarditis. However, severe scrub typhus was commonly treated in infection specialist. ST change in ECG cannot be detectable because ECG cannot be routinely examined on the infection department in the ICU.
Scrub typhus myocarditis can be a subclinical manifestation of cardiac involvement; however, some patients with scrub typhus might have undetected mild myocarditis. The present diagnosis of acute myocarditis was not easy [18]. At first, all cases confirmed by endomyocardial biopsy were reported in 1991 in which heart failure occurred 3 months after an acute febrile illness [19].
Early detection of scrub typhus myocarditis is crucial before patients develop cardiogenic shock which depends on whether hemodynamic support and the appropriate antibiotics are administered early on. The mortality rate of acute myocarditis in a previous study was high; however, cardiac recovery was excellent in those who survived with proper treatment [12].
The investigation that might have helped was the abnormal ST-segment change in the ECG, cardiac troponin, echocardiogram and cardiac magnetic resonance. The gold standard for diagnosing myocarditis is myocardial biopsy. However, in a real practice, infection with scrub typhus has been mainly treated in department of infectious diseases. Cardiologic evaluation could not routinely be evaluated in an infection state; additionally, magnetic resonance images are not easily available and cost-effective even though highly specific for myocarditis [7,20].
In our study, PAF could be a predictor for acute myocarditis which provides a timely and appropriate diagnosis through the use of an echocardiography and reduces the mortality rate of scrub typhus.
In study limitations, first, this was a retrospective study. Selection of severe and complicated scrub type was dependent on the physicians, and the baseline characteristics of each group were not matched. However, all consecutive patients in the ICU undergoing cardiologic evaluation were included as severe and complicated scrub typhus. Second, the duration of illness for scrub typhus was unknown before ICU admission. Third, scrub typhus myocarditis was diagnosed with both a cardiac biomarker and echocardiogram and not by cardiac tissue biopsy. All the patients had no serial ECG, and the clinical information on the duration and frequencies of PAF for each patient were missing.
In conclusion, our study suggests that PAF could be a predictor of acute myocarditis as a complication of scrub typhus and could be used for cardiac evaluation which includes cardiac biomarkers and an echocardiography for proper diagnosis and management in the ICU.
KEY MESSAGE
1. The incidence of acute myocarditis with severe scrub typhus may be higher than previously reported.
2. Patients with high bilirubin and PAF are at increased risk of acute myocarditis with scrub typhus. These patients warrant closer follow-up and urgent echocardiogram would be needed.
Notes
No potential conflict of interest relevant to this article was reported.
Supplementary Materials
Demographic and clinical characteristics and outcome of patients with scrub typhus myocarditis
Baseline clinical characteristics of patients with scrub typhus with and without atrial fibrillation admitted to the intensive care unit