KRAS G12C mutation as a poor prognostic marker of pemetrexed treatment in non-small cell lung cancer
Article information
Abstract
Background/Aims
The predictive and prognostic value of KRAS mutation and its type of mutations in non-small cell lung cancer (NSCLC) are controversial. This clinical study was designed to investigate the predictive value of KRAS mutations and its mutation types to pemetrexed and gemcitabine based treatment.
Methods
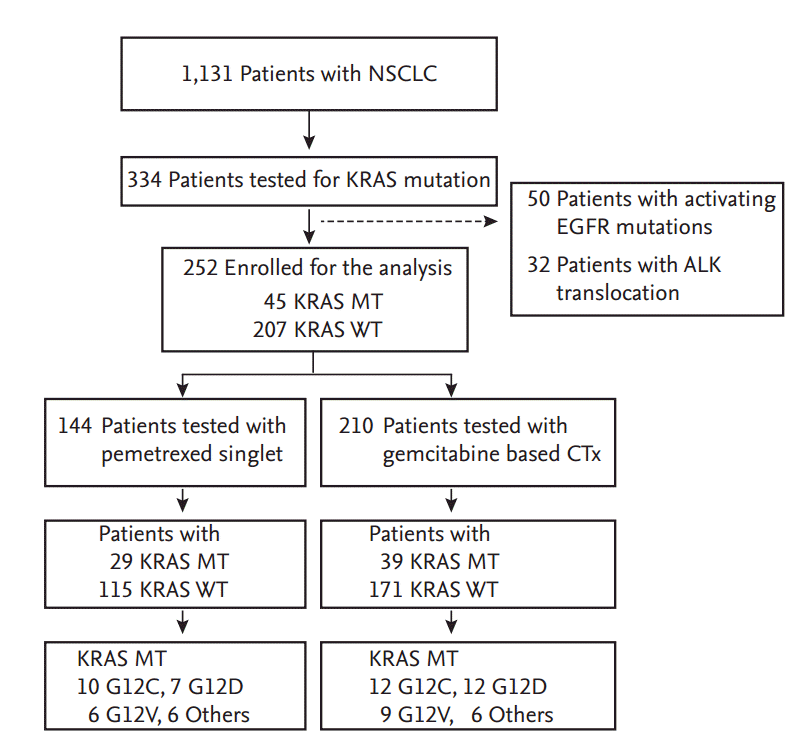
Advanced NSCLC patients tested for KRAS mutation (n = 334) were retrospectively reviewed and 252 patients with wild type epidermal growth factor receptor and no anaplastic lymphoma kinase fusion were enrolled for the analysis. KRAS mutations were observed in 45 subjects with mutation type as followed: G12C (n = 13), G12D (n = 12), G12V (n = 12), other (n = 8). Response rate (RR), progression-free survival (PFS), and overall survival (OS) of pemetrexed singlet and gemcitabine based chemotherapy were analysis.
Results
Age, sex, performance status were well balanced between subjects with or without KRAS mutations. No difference was observed in RR. Hazard ratio (HR) of PFS for pemetrexed treated subjects with G12C mutation compared to subjects with KRAS wild type was 1.96 (95% confidential interval [CI], 1.01 to 3.79; p = 0.045), but other mutations failed to show clinical significance. By analysis done by PFS, compared to the subjects with transition mutation, HR was 1.48 (95% CI, 0.64 to 3.40; p = 0.360) for subjects with transversion mutation on pemetrexed treatment and 0.41 (95% CI, 0.19 to 0.87; p = 0.020) for subjects treated with gemcitabine based chemotherapy. No difference was observed in OS.
Conclusions
In this study, different drug sensitivity was observed according to the type of KRAS mutation. NSCLC subpopulations with different KRAS mutation type should be considered as different subgroups and optimal chemotherapy regimens should be searched in further confirmative studies.
NTRODUCTION
Non-small cell lung cancer (NSCLC), which constitutes 85% of lung cancer [1], is a leading cause of cancer related mortality [2]. However, prognosis of NSCLC differs based on its molecular profiles, and individualized therapy using targeted agents against activated signal pathways demonstrated promising prognosis [3,4]. Subpopulations without actionable molecular targets are still candidates of conventional cytotoxic chemotherapy and comparably poor prognosis is expected.
Approximately 54% of NSCLC patients have been identified with epidermal growth factor receptor (EGFR), KRAS mutations, or anaplastic lymphoma kinase (ALK) fusion usually mutually exclusive [5-7]. Among the mutations, KRAS mutations occur up to 30% of NSCLC patients [8], and higher mutation rates were observed in patients with previous history of smoking and adenocarcinoma histology type [9]. Former or current smokers were more likely to have transversion mutations (G→T or G→C), in contrast to transition mutation (G→A) being frequently observed in non-smokers [10]. More in detail, the majority of missense mutations occur primarily at codon 12 or 13, causing single amino acid substitutions at residues G12 or G13 [11].
The clinical implication of KRAS mutations as a predictive and prognostic marker is controversial. Some reports demonstrated increased overall survival (OS) hazard ratio (HR) in KRAS mutants compared with wild type [12-14], but others have failed to demonstrate consistent results [15,16]. Subgroup analysis done by location of amino acid substitution, codon 12 and codon 13, also failed to show significant difference in OS and response to chemotherapy [17]. As a predictive marker for response to chemotherapy, a recent preclinical study done by NSCLC cell line identified enhanced dependency with anti-folate treatment by overexpression of genes within folate metabolism pathways in KRAS mutated cancer cells [18]. However, this result was also contrary to previous retrospective clinical studies [19,20].
Due to failure of small molecules targeting downstream of KRAS signal pathway [21,22], conventional cytotoxic chemotherapy is still recommended for NSCLC patients with KRAS mutations. Regarding the different KRAS epidemiology of each mutation among smokers and non-smokers, our study was designed to compare response of chemotherapy based on each type of KRAS mutation.
METHODS
Study population
NSCLC patients (n = 1,131) treated with conventional cytotoxic chemotherapy from July 2002 to December 2014 in Seoul National University Hospital (SNUH) were retrospectively reviewed. Out of 1,131 patients, 334 patients were tested for KRAS mutation status by direct DNA sequencing method. Test for KRAS mutation was conducted depends on availability of test and clinician’s preference. Some patients were also tested for pre-screening purpose to decide eligibility of clinical trial enrollment. A total of 50 patients with activating EGFR mutations or unknown EGFR mutation status and 32 patients with ALK fusion were excluded. Analysis was conducted in two different groups. The first group consisted of 144 subjects treated with pemetrexed singlet chemotherapy and the second group consisted of 210 patients treated with gemcitabine based cytotoxic chemotherapy (Fig. 1). This study was approved by the SNUH Institutional Review Board (IRB No. 1412-103-634) and conducted in accordance with the World Medical Association’s Declaration of Helsinki. Requirement for informed consent was waived.
Data collection
Each patient’s medical record was retrospectively reviewed and medical history, pathology data, treatment history, imaging data, and genetic mutation results were acquired. Smokers were categorized as current former, or never smokers. Direct DNA sequencing method or peptide nucleic acid clamping test from tumor tissue was done to determine KRAS and EGFR mutation status. ALK translocation was tested by immunohistochemistry test. KRAS mutations were recorded more in detail by type of mutation, and categorized in four different groups: G12C, G12D, G12V, and mutations with other amino acid substitutions. Other mutations were Q22R, G13D, G12A, and G12F. Treatment response was evaluated by comparing post-treatment computed tomography (CT) to pre-treatment CT and in accordance to the Response Evaluation Criteria in Solid Tumor (RECIST) version 1.1. Survival data were collected from the Korean death registry and acquired from the Korean National Statistical Office.
The primary end point of our study was assessing the HR of each KRAS mutation by amino acid substitution to pemetrexed singlet treatment and gemcitabine based chemotherapy.
Statistical analysis
Baseline demographics of patients were analyzed with descriptive statistics between subjects with KRAS mutations and KRAS wild type. Additional analysis was conducted by subjects with four different categories by amino acid substitutions versus subjects with KRAS wild type, subjects with transition mutation and subjects with transversion mutation. Response rate by RECIST was compared with either chi-square test or Fisher exact test. HRs of progression-free survival (PFS) and OS were analyzed by Cox-proportional hazard regression analysis. Kaplan-Meier curves were used to portray the failure of treatment and calculate median PFS. Log-rank test was used to test the difference between the curves.
PFS of each patient was calculated from the date of initiation of palliative chemotherapy to the date of cancer progression or all-cause mortality. OS was calculated from the date of palliative setting of treatment to the date of cancer related mortality.
All results with two-sided p value less than 0.05 were considered as significant and all the data were analyzed by STATA version 12.1 (StataCorp., College Station, TX, USA)
RESULTS
Characteristics of study population
Baseline clinical demographics of the total 252 patients are listed in Table 1. All subjects with KRAS mutations and KRAS wild type are well balanced. KRAS mutations were common in adenocarcinoma (86.7%) as previously reported [9], but no statistical difference was observed by smoking status (p = 0.866). Eastern Cooperative Oncology Group (ECOG) performance status (PS) was categorized by subjects with ECOG PS 0 and 1, and the subjects with poor performance status, ECOG PS 2, 3, and 4.
Approximately 95% of gemcitabine based chemotherapy was applied as first line chemotherapy. Pemetrexed singlet was applied as second line chemotherapy in 77.1% of patients. Detailed description of subjects’ profile by each type of KRAS mutations are shown in Table 1.
Response rate of treatment
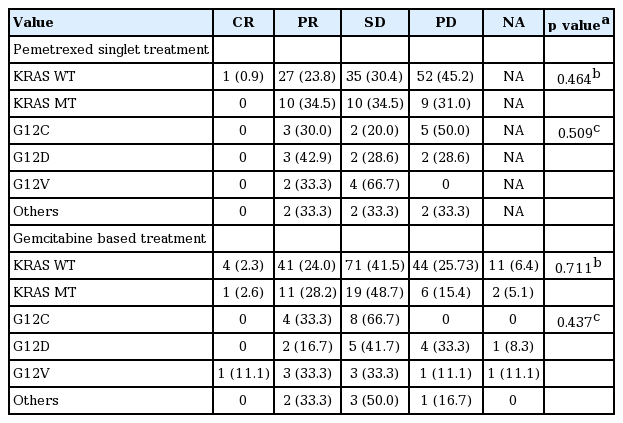
Response rate of pemetrexed singlet treated patients (n = 144) between subjects with KRAS mutation and wild type failed to demonstrate significant difference (p = 0.464). Subgroup analysis done by the four categories showed no difference in response rate (p = 0.509).
All gemcitabine based chemotherapy regimens were combined (n = 210) and analyzed for difference in response rate. In the same manner, difference between KRAS mutation and wild type (p = 0.711), and difference between each type of KRAS mutation (p = 0.437) were insignificant (Table 2).
Hazard ratio of progression-free survival and overall survival
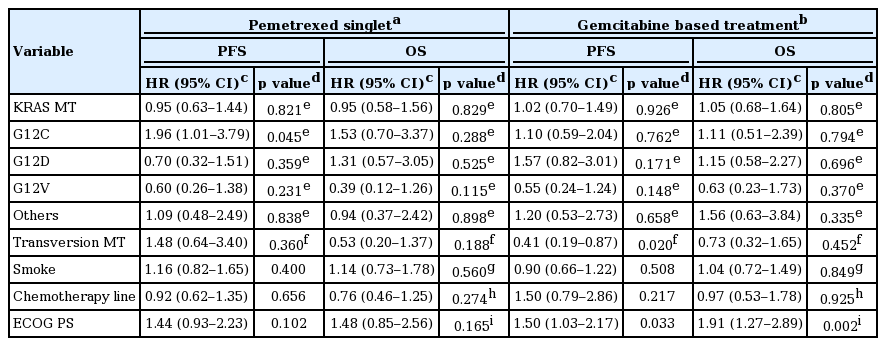
The relationship between KRAS mutations and PFS are portrayed by Kaplan-Meier curves in Fig. 2. PFS of KRAS mutant subjects treated with pemetrexed singlet (n = 144) were compared to subjects with KRAS wild type and HR was calculated as 0.95 (95% confidential interval [CI], 0.63 to 1.44; p = 0.821). No difference in HR was observed in OS (HR, 0.95; 95% CI, 0.58 to 1.56; p = 0.829). Subgroup analysis by each amino acid substitution showed significantly shorter PFS in subjects with G12C mutation compared to KRAS wild type subjects (HR, 1.96; 95% CI, 1.01 to 3.79; p = 0.045). Other substitutions failed to show significant difference.
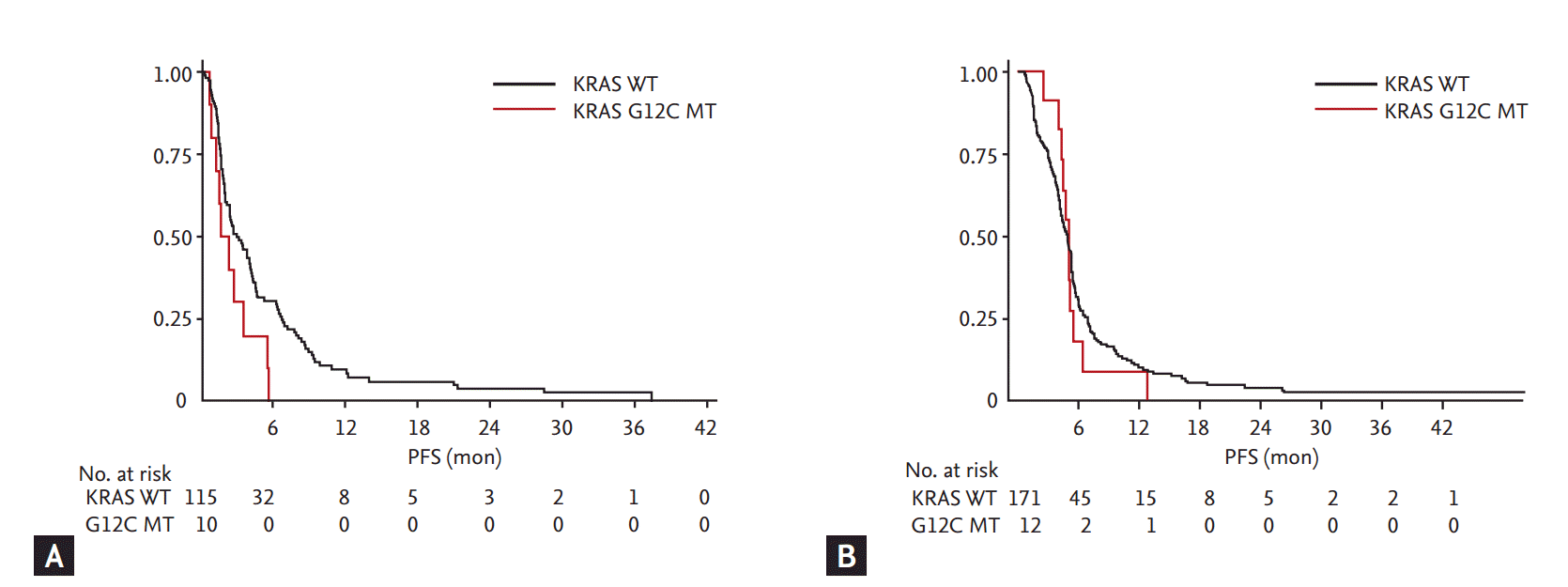
Kaplan-Meier curves of progression-free survival (PFS). PFS curve plotted by subjects treated by pemetrexed singlet. (A) Subjects with KRAS wild type (WT) and G12C mutations (MTs) ([median PFS, 2.9 months; 95% confidential interval (CI), 2.3 to 4.0] vs. [median PFS, 1.6 months; 95% CI, 0.6 to 3.4], p = 0.040); PFS curve plotted by subjects treated by gemcitabine based chemotherapy. (B) Subjects with KRAS wild type and G12C mutations ([median PFS, 4.8 months; 95% CI, 4.2 to 5.2] vs. [median PFS, 5.0 months; 95% CI, 4.0 to 5.3], p = 0.761).
Same methods were applied to subjects treated with gemcitabine based chemotherapy (n = 210). Notably, subjects with transversion mutation showed decreased HR of PFS (HR, 0.41; 95% CI, 0.19 to 0.87; p = 0.020) compared to subjects with transition mutation (Table 3, Fig. 3). No statistical significance was observed on survival analysis done by OS. More in detail, when subgroup analysis conducted with subjects treated with 1st line gemcitabine based chemotherapy (n = 199), transversion mutation showed HR 0.38 (95% CI, 0.18 to 0.81; p = 0.012).
Cox-proportional hazard ratios of pemetrexed singlet treatment and gemcitabine based treatment by each type of KRAS mutation
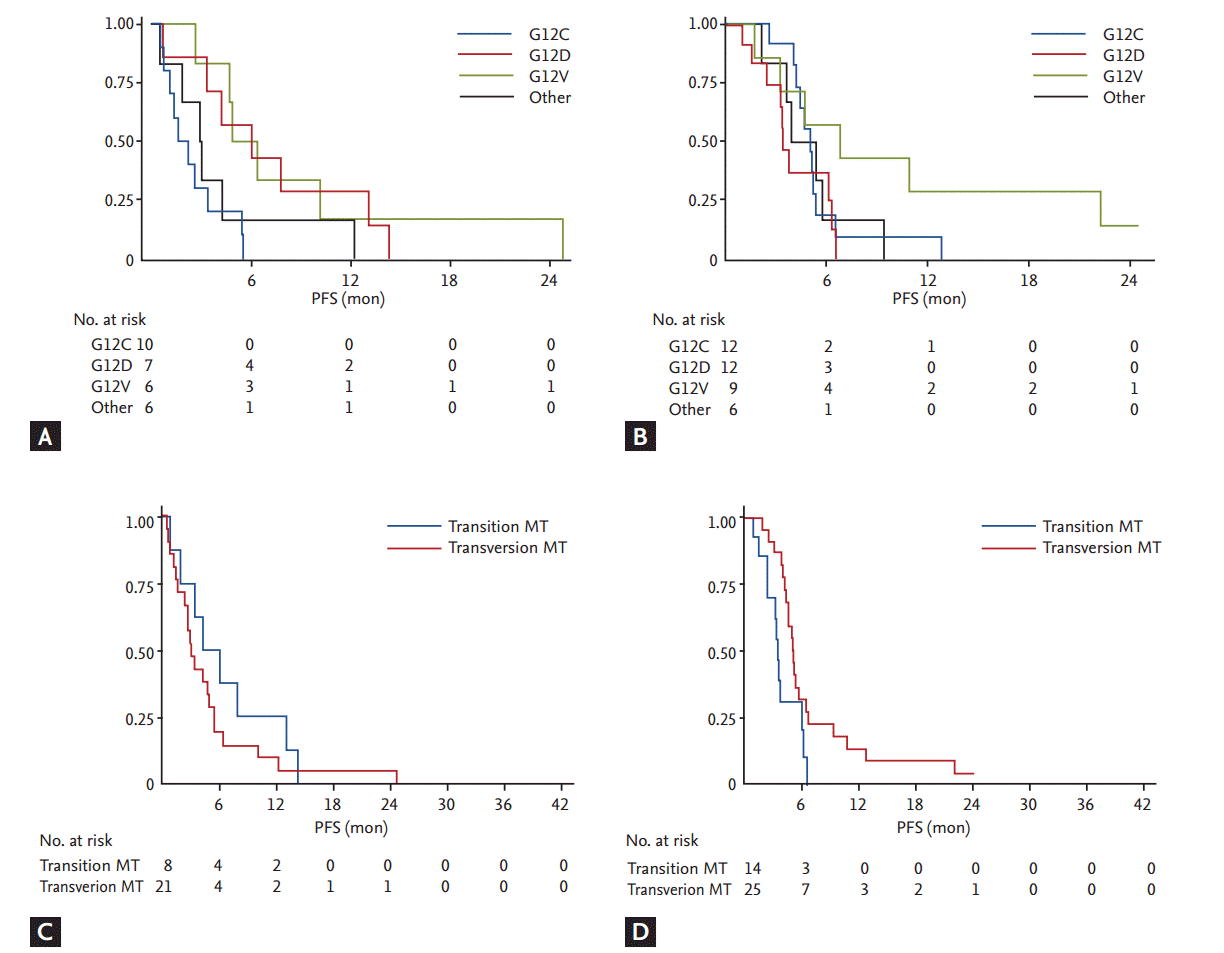
Kaplan-Meier curves of progression-free survival (PFS). (A, B) PFS curve plotted by each type of KRAS mutation (MT): (A) subjects with pemetrexed singlet treated; (B) subjects with gemcitabine based chemotherapy treated. (C, D) PFS curve plotted by transition mutation or transverse mutation: (C) subjects with pemetrexed singlet treatment; (D) subjects with gemcitabine based chemotherapy treatment.
DISCUSSION
Despite the fact that KRAS mutations are common molecular changes in NSCLC, its utility as a clinical predictive marker was disappointing due to the controversial results [23]. Moreover, its role as a predictive marker of cytotoxic chemotherapy had not been established. However, looking into more detail epidemiology of KRAS mutation, observation that KRAS transition mutation (G12D mutation) was more common in never smokers and transversion mutation (G12C, G12V mutation) in smokers corresponded with the controversial results [10,24,25]. In a perspective view, shorter OS in NSCLC with smoking history were also observed in NSCLC patients without considering mutational status [19]. Summarizing the above information, we have hypothesized and approached each type of KRAS mutation as a confounder of previous studies and as an independent variable in our study.
In this study, we have investigated the predictive value of each type of KRAS mutation to response from pemetrexed singlet treatment and gemcitabine based combination chemotherapy. As a result, subjects with G12C mutation are likely to demonstrate shorter PFS to pemetrexed treatment, and subjects with transition mutation (purine to pyrimidine or pyrimidine to purine mutation) are also expected to show shorter PFS to gemcitabine based treatment compared to wild type.
Similar result is evidenced in previous studies. The RASCAL (The Kirsten ras in-colorectal-cancer collaborative group) II study done with colon cancer patients showed that G12V mutation had poor prognosis in both PFS (p < 0.004) and OS (p = 0.008) [26]. In the BATTLE (The Biomarker-integrated Approaches of Targeted Therapy for Lung Cancer Elimination) trial conducted with NSCLC patients, initial analysis done by overall presence of KRAS mutation failed to present clinical value for KRAS mutations [27], in which subgroup analysis comparing subjects with G12C or G12V KRAS mutations to subjects with wild type or other type KRAS mutations showed shorter PFS (p = 0.046) [28]. Molecular structure modeling also confirmed that KRAS protein with G12C changes are likely to activate the Ral (RAS-like) protein signaling pathway, unlike KRAS protein with G12D changes that are prone to activate RAS signaling cascade by activating phosphatidylinositol 3-kinase signaling pathways [28].
Different biology has been validated previously, but differences in drug sensitivity are unknown. A study conducted with NSCLC cell line show that G12C mutation is associated with a reduced response to cisplatin and increased response to pemetrexed. However, G12V mutation was sensitive in cisplatin and decreased in response to pemetrexed. No difference was observed between wild type and different KRAS mutations in response to gemcitabine [29]. But adverse results showing no correlation on folate receptor and KRAS were also reported [30]. In human studies, results were also controversial in retrospective studies comparing pemetrexed sensitivity by type of KRAS mutation. Although all studies have been reported in median OS, same tendency of poor respond to pemetrexed in subjects with G12C were demonstrated in a report [31] but another showed the opposite result [32].
Current guidelines for advanced NSCLC patients without activating mutations, including KRAS mutations, recommend starting treatment with pemetrexed, gemcitabine or taxane based treatment [33]. Due to limitation in our study, direct clinical application cannot be recommended, but it may be suggested that different treatment approach by type of KRAS mutation may be beneficial. G12C mutant subjects are likely to show early failure of pemetrexed treatment, and better response is expected in patients with G12D and G12V mutations. In the same manner, subjects with transversion KRAS mutations are expected to better respond to gemcitabine based treatment compared to subjects with transition mutations (Table 3). Statistical significance was not satisfied for all the categories and direct comparison between regimens were not allowed, nonetheless, different tendency in response were observed by each mutation.
Our study has limitations. Subjects with KRAS mutations were limited in number as well as heterogeneous chemotherapy regimens were applied. Hence, statistical power for subgroup analysis was limited. Further pooled analysis should be conducted by each type of KRAS mutation to validate our results. Currently, pemetrexed singlet treatment is not routinely used for NSCLC patients. However due to the national insurance reimbursement policy in South Korea, we were allowed to analyze subjects treated with singlet pemetrexed which is meaningful because direct clinical effect of pemetrexed by each mutation without other confounding drug effect could be considered. Although our results have limitation to suggest clear clinical implication for patients with KRAS mutations, to our knowledge, this study is the first clinical study to test drug sensitivities by each mutation.
In conclusion, we have demonstrated G12C KRAS mutation showed reduced PFS to pemetrexed singlet treatment and value of transversion KRAS mutation as a good predictive marker of gemcitabine based chemotherapy. Further studies are warranted for confirmation.
KEY MESSAGE
1. Non-small cell lung cancer subpopulations with different KRAS mutation showed different sensitivity to drug.
2. In patients with G12C KRAS mutation showed reduced response to pemetrexed treatment.
3. Transversion mutation showe good response to gemcitabine based chemotherapy.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by a grant from the Innovative Research Institute for Cell Therapy, Republic of Korea (A062260).