Practical management of peripartum cardiomyopathy
Article information
Abstract
Peripartum cardiomyopathy (PPCM) is an idiopathic cardiomyopathy that causes systolic heart failure (HF) in previously healthy young women. Despite latest remarkable achievement, unifying pathophysiologic mechanism is not well established. Considering close temporal relationship to pregnancy, the recent prolactin theory is promising. Abnormal short form of 16-kDa prolactin may be produced in the oxidative stress milieu, show anti-angiogenic effect and damage cardiovascular structure in late pregnancy. Future study is needed to determine whether abnormal prolactin system is useful as a biomarker for diagnosis and therapy of PPCM. Diagnosis is made based on the finding of left ventricular systolic dysfunction after excluding other causes of HF. A multidisciplinary team approach is essential for acute HF, antepartum, labor and postpartum care. Recovery from left ventricular dysfunction is critical for prognosis. As PPCM can recur and cause serious clinical events, subsequent pregnancy is not recommended. This review focuses on the practical management of PPCM.
INTRODUCTION
Cardiovascular disease is an important cause of mortality and morbidity during pregnancy. Peripartum cardiomyopathy (PPCM) is a severe form of idiopathic cardiomyopathy, affecting previously healthy young women during late pregnancy or postpartum, which can be life threatening. The actual incidence of PPCM is unknown and highly variable reports exist across a variety of countries, with 1:299 live births in Haiti, 1:1,000 live births in South Africa, and 1:1,149 to 4,000 live births in the USA [1]. Risk factors for PPCM include advanced maternal age, multiparity, multiple pregnancies, gestational hypertension or preeclampsia, and prolonged tocolysis [2]. As women are bearing children at later ages, the incidence of PPCM may increase. We here summarize the current knowledge concerning the practical management of PPCM.
DEFINITION OF PERIPARTUM CARDIOMYOPATHY
In 2010, the Working Group on PPCM of the European Society of Cardiology defined PPCM as, ‘an idiopathic cardiomyopathy presenting with heart failure (HF) secondary to left ventricular (LV) systolic dysfunction towards the end of pregnancy or in the months following delivery, where no other cause of HF is found.’ It is a diagnosis of exclusion. While the LV may not be dilated, the ejection fraction (EF) is nearly always reduced below 45% [2].
NORMAL CHANGES DURING PREGNANCY
Women experience dramatic hemodynamic changes during pregnancy, which include a profound decrease of systemic vascular resistance and corresponding increases in blood volume and cardiac output [3]. Such changes start as early as the first trimester. Hormone levels vary significantly during gestation and are critical in maintaining cardiovascular adaptation during pregnancy [4]. Pregnancy hormones such as relaxin, progesterone, and estrogen induce vascular relaxation. The activated renin-angiotensin-aldosterone system induces salt and water retention, which helps maintain blood volume. Moreover, cardiac volume overload induces physiologic cardiac hypertrophy. Progesterone causes protein synthesis and cardiomyocyte hypertrophy and induces systemic angiogenesis. Such pregnancy-related cardiac adaptation processes may be altered by cytokines in the pathological setting of significant hypertension [5] or PPCM [4].
ETIOLOGY AND PATHOPHYSIOLOGY OF PERIPARTUM CARDIOMYOPATHY
The etiology and precise pathophysiological mechanisms of PPCM remain unknown. Several hypotheses suggest that multifactorial processes are involved [6-13].
Genetic factors
Although PPCM is classified as a nonfamilial, nongenetic form of cardiomyopathy [6], the tendency toward a familial occurrence of PPCM suggests a genetic propensity [6-9]. The initial manifestation of PPCM may present as familial dilated cardiomyopathy (DCM) [8]. Many genetic variants of PPCM are common in familial DCM, suggesting an overlap in the clinical spectrum of PPCM and DCM [9]. Genetic variations may be responsible for the geographic or ethnic variations in the incidence of PPCM.
Myocardial inflammation
Previous research has suggested a role of inflammation in PPCM. A high rate of active myocarditis was reported in endomyocardial biopsy PPCM samples [10]. One report showed viral genomes with interstitial inflammation in 31% of PPCM cases [11]. High concentrations of tumor necrosis factor α, interferon γ, interleukin 6, C‑reactive protein, and Fas/apoptosis antigen 1 were obtained in PPCM [12]. Inflammatory cytokines may activate unfavorable cardiac adaptations during pregnancy and are associated with cardiac fibrosis. However, the exact cause of myocardial inflammation in PPCM is unknown.
Angiogenic imbalance
Angiogenic balance is inclined toward angiogenesis during normal pregnancy. However, in late gestation, the placenta secretes antiangiogenic substances such as vascular endothelial growth factor inhibitors. Excess antiangiogenic signaling induces a cardiac-specific or systemic antiangiogenic environment in PPCM [13]. The perspective of PPCM as a vascular disease is in agreement with PPCM developing in late gestation and may contribute to why pre-eclampsia and multiple gestations are associated with PPCM.
Oxidative stress and the prolactin theory
Oxidative stress increases during normal pregnancy. Maternal metabolic changes including insulin resistance and a metabolic shift toward fatty acids tend to increase oxidative stress. The level of oxidative stress is more exaggerated in PPCM compared with normal pregnancy. In mice, deletion of the signal transducer and activator of transcription 3 gene, which are essential for cardioprotective effects via reactive oxygen species scavenging, results in the production of pathologic 16-kDa prolactin [14]. While full-length prolactin has proangiogenic effects, the 16-kDa cleaved form has antiangiogenic and proapoptotic properties; therefore, it can result in the destruction of cardiac and vascular tissue. The prolactin theory is attractive in that the majority of PPCM cases occur during the limited peripartum period.
CLINICAL PRESENTATION
Although multiparity is an important risk factor, PPCM occurs in the young primigravida in up to one third of cases [15]. Clinical presentation resembles DCM with systolic HF including fatigue, exertional dyspnea, orthopnea, paroxysmal nocturnal dyspnea, leg edema, neck vein engorgement, pulmonary crackles, hepatic congestion, and third heart sound [2]. Early symptoms are frequently confused with physiologic phenomena of normal pregnancy. In most cases, symptoms develop within 4 months after delivery. Antepartum presentation occurs in less than 10% of cases. The severity of symptoms is highly variable from New York Heart Association functional class I to IV; however, class III or IV symptoms are most common [15]. Moreover, an asymptomatic latent form has previously been described [16]. The clinical course is highly variable, from rapid progress within days to an indolent course over months. Life-threatening complications include refractory HF, cardiogenic shock, severe ventricular arrhythmia, multiorgan failure, thromboembolism, and even death. Patients with severe LV dysfunction are at high risk of LV thrombosis and systemic embolism [17].
DIAGNOSIS
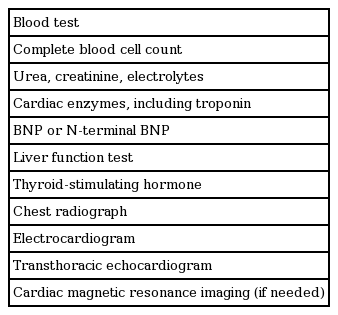
Diagnosis is often delayed because symptoms of PPCM are not specific, particularly in late pregnancy. PPCM should be considered in all peripartum women with signs and symptoms of HF or those with delayed recovery to a pre-pregnant state (Fig. 1). Complete family history should be obtained to identify familial patterns of cardiomyopathy. Chest X-ray can show cardiomegaly, pulmonary congestion, and pleural effusion. While electrocardiogram is not specific for PPCM, several findings including non-specific ST-T wave abnormality, QT interval prolongation, QRS widening, LV hypertrophy, and atrial fibrillation, may indicate this pathologic process and urge cardiac evaluation. The following laboratory tests are necessary, although they are not specific for PPCM: complete blood cell count, blood urea, creatinine, electrolytes, liver function test, thyroid stimulating hormone, cardiac troponin, and brain-type natriuretic peptide (BNP) (Table 1). Recent experimental studies suggest some biomarkers might be specific for PPCM; the 16-kDa prolactin, microRNA-146a, and soluble fms-like tyrosine kinase-1 have been proposed, although their diagnostic value in clinical practice needs verification [13,14,18]. Endomyocardial biopsy is warranted in some cases to exclude the inflammatory etiology of acute HF.

Flow chart for suspected peripartum cardiomyopathy (PPCM). HF, heart failure; CBC, complete blood count; BUN, blood urea nitrogen; ECG, electrocardiogram; BNP, brain-type natriuretic peptide; NT-proBNP, N-terminal pro-brain natriuretic peptide; MRI, magnetic resonance imaging.
BNP or N-terminal pro-brain natriuretic peptide
BNP or N-terminal pro-brain natriuretic peptide (NTproBNP) levels are helpful to screen potential PPCM patients. BNP or NT-proBNP plasma concentrations have high sensitivity to include, and high specificity to preclude, HF. Despite significant hemodynamic stress, the blood concentration of BNP or NT-proBNP does not increase during normal pregnancy [19]. However, BNP or NT-proBNP levels rise in heart diseases including pre-eclampsia or eclampsia, congenital heart disease with structural defects, and various forms of cardiomyopathy. High BNP or NT-proBNP values before or during pregnancy predict adverse maternal events in pregnant women. Normal BNP levels (≤ 100 pg/mL) showed a very high negative predictive value for adverse maternal events even in pregnant women with heart disease. NT-proBNP levels > 300 pg/mL prior to pregnancy was associated with complications during the peripartum period in women with DCM.
Echocardiography
Echocardiography is central to the diagnosis of PPCM. LV dimension, geometry, and systolic and diastolic function can be assessed by echocardiography. PPCM is diagnosed using LV systolic dysfunction. The original echocardiographic criteria for PPCM were strict: LV end-diastolic dimension > 2.7 cm/m2 and M-mode fractional shortening < 30% or LV EF < 45% [20]. In the latest guideline notes, LV dilation is not mandatory for PPCM diagnosis [17]. Moreover, echocardiography is useful for excluding differential diagnoses. Significant diastolic dysfunction with preserved systolic function suggests hypertensive heart diseases such as preeclampsia rather than PPCM. Moreover, marked right ventricular dysfunction is suggestive of pulmonary embolism, while regional wall motion abnormality of the LV is often observed in myocarditis or acute coronary syndrome.
Cardiac magnetic resonance imaging
Cardiac magnetic resonance imaging (MRI) can complement echocardiography, particularly in patients with suboptimal echocardiographic images. Global and segmental LV contraction can be analyzed and ischemic heart disease or acute myocarditis can be revealed as alternate diagnoses. Late gadolinium enhancement (LGE) levels provide valuable prognostic information in ischemic and non-ischemic heart disease [21,22], although the clinical significance of LGE in PPCM is not established. In PPCM, extensive LGE is likely to be a myocarditis-like transitory feature and is not necessarily an adverse predictor for long-term LV recovery [23]. As gadolinium crosses the placenta, the benefits versus risks for administration should be carefully weighed in antepartum women [24].
Endomyocardial biopsy
Although the literature suggests that myocardial inflammation would be involved in the pathogenesis of PPCM, endomyocardial biopsy yields uncertain results. As there are no histological criteria to confirm a PPCM diagnosis, endomyocardial biopsy is not routinely recommended in the current guidelines [2]. Endomyocardial biopsy can be considered to preclude the diagnosis of acute myocarditis or other forms of cardiomyopathy when the clinical course deteriorates during standard medical treatment.
Working diagnostic criteria for PPCM
In clinical practice and research, the diagnostic criteria used, which are composed of the classic definition of PPCM and echocardiographic criteria, are as follows: (1) development of HF in the last month of pregnancy or within 5 months after delivery; (2) LV systolic dysfunction (LV EF < 45% by echocardiography); (3) no identifiable cause for HF; and (4) no recognized heart disease before the last month of pregnancy (Table 2) [20,25,26]. All four criteria must be met for PPCM diagnosis. Current diagnostic criteria help to exclude pre-existing DCM and to avoid the over-diagnosis of PPCM. Because the exact mechanism of PPCM is unknown, the diagnostic criteria do not reflect the pathophysiological aspect but, rather, emphasize the limited duration of presentation. Future studies may change the diagnostic criteria.
DIFFERENTIAL DIAGNOSIS
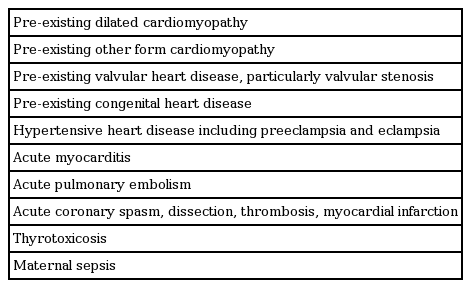
PPCM is a diagnosis of exclusion. Conditions that should be considered are listed in Table 3.
Pre-existing or familial dilated cardiomyopathy
Previous familial and genetic studies have noted significant overlap in DCM and PPCM [8,9]. PPCM resembles DCM in clinical presentation and cardiac imaging. However, PPCM is a different disease and is not the reversible form of DCM. The timeframe of the clinical course is an important component of differential diagnosis. While HF symptoms develop in the second trimester in the majority of cases of DCM, when hemodynamic stress increases rapidly ('early onset pregnancy-associated cardiomyopathy'), the typical form of PPCM develops postpartum (‘late onset pregnancy-associated cardiomyopathy’). In women with DCM of mild LV dysfunction, HF symptoms may develop only in the third trimester when hemodynamic stress reaches a peak [27]. In such cases, current approaches are unable to differentiate the two diseases. The pathophysiological mechanisms are also different in PPCM and DCM. PPCM develops under the critical influence of temporal hormonal changes, although the exact underlying mechanism remains unknown.
Acute myocarditis
Viral or other types of acute myocarditis may occur during the peripartum period. Rapidly progressing acute HF mimics PPCM. The hesitation with endomyocardial biopsy includes low yield and unease performing an invasive procedure in pregnant women. Cardiac MRI can be an alternative when there is a high likelihood of acute myocarditis.
Preeclampsia with heart failure
Severe hypertension including preeclampsia may cause critical diastolic dysfunction and obvious HF in pregnant women. Pregnancy-related blood volume overload precipitates cardiac decompensation in hypertensive heart disease and preeclampsia, which may be confused with PPCM. Echocardiography helps to differentiate the two diseases. LV EF is low in PPCM but is preserved in preeclampsia. Significant diastolic dysfunction and findings suggestive of elevated left atrial filling pressure indicate preeclampsia instead of PPCM.
MANAGEMENT
Treatment of PPCM follows the standard treatment for other types of systolic HF. Medications require adjustments before delivery, according to fetal toxicity. Goals are to improve hemodynamic status, to minimize symptoms of HF, and to optimize long-term outcomes. Antepartum fetal wellbeing is a crucial outcome. A team approach with collaboration between cardiologists, obstetricians, pediatricians, and anesthesiologists is essential.
Management of stable patients
The goal of management is to improve hemodynamic status, minimize symptoms, and obtain the best fetal outcome [2]. General recommendations include a low sodium diet, fluid restriction, and light activity. When the patient is stable, oral HF medication is the mainstay of treatment (Table 4). Special attention is required to ensure fetal safety and to monitor drug excretion during breastfeeding.
Angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin-receptor blockers (ARBs) are major vasodilators that effectively decrease afterload. However, these are contraindicated during pregnancy because of serious fetal toxicity. A combination of hydralazine and nitrates can be used as a safe alternative. Benazepril, captopril, and enalapril are safe in nursing women [17]. ARBs are used if ACEIs are not tolerated. Due to the lack of safety data, ARBs should be avoided during breastfeeding. The mineralocorticoid receptor antagonist, spironolactone, is also contraindicated during pregnancy and breastfeeding.
β-Blockers are another major drug used in PPCM management. As β-blockers reduce mortality in patients with systolic HF, they are recommended for all women with PPCM for at least 6 months after full recovery [3]. The clinical response to β-blockers may vary because of genetic polymorphisms [28]. Special caution is needed in the early stage of acute decompensated HF as β-blockers can decrease LV contraction and systemic perfusion. Due to concern regarding the influence of β-blockers on uterine tone, β1-selective blockers are preferred before birth. For nursing women, metoprolol is the best-studied agent [17]. Caution is needed because β-blockers can result in hypoperfusion of the maternal cardiac and uteroplacental circulation. Fetal wellbeing should be monitored as β-blockers may cause fetal bradycardia.
Diuretics are indicated for women with pulmonary congestion and dyspnea. Caution is warranted because diuretics may decrease placental blood flow. Oral thiazides can be used safely during pregnancy and breastfeeding. Furosemide can be used if the response is suboptimal. Digoxin can be considered in pregnant women with HF symptoms and a low EF. In addition to hypercoagulability during normal pregnancy, PPCM is a notable prothrombotic condition; LV thrombus, deep vein thrombosis, and pulmonary embolism are common. The risk of LV thrombosis is particularly high in those with severe LV dysfunction. To prevent systemic thromboembolism, anticoagulation is recommended for women with PPCM and EF < 35% [2]. Low molecular weight heparin is preferred during pregnancy and it can be switched to warfarin postpartum.
Bromocriptine, a dopamine antagonist that inhibits prolactin secretion, is a recent experimental candidate for PPCM treatment [14,17]. In a pilot study, bromocriptine prevented clinical deterioration when added to standard HF therapy [29]. An ongoing clinical trial will address whether bromocriptine can be recognized as the standard treatment for PPCM [30]. Due to the concern of thrombosis including myocardial infarction, an anticoagulant should be used during bromocriptine treatment. The medications used for PPCM treatment are summarized in Table 4.
Management of decompensated heart failure
The goal of management is to establish the diagnosis, stabilize hemodynamic status, relieve symptoms, and ensure fetal safety. The optimal status of ‘ABC’ (airway, breathing, and circulation) should be secured [2]. Endotracheal intubation is needed in cases of impending respiratory failure; however, non-invasive ventilation with a positive end-expiratory pressure of 5 to 7.5 cm H2O can be tried instead. Supplemental oxygen may be provided to maintain arterial oxygen saturation ≥ 95%. Monitoring with pulse oximetry is mandatory and frequent arterial blood gas analysis is indicated until breathing is stabilized. Electrocardiography and cardiac monitoring of blood pressure (BP) and heart rate should be performed continuously. Invasive monitoring is needed in patients with hemodynamic instability [31]. An intra-arterial line can be used to provide accurate BP readings. BP measured with a non-invasive cuff is unreliable in situations of intense hypotension, arrhythmias, increased systemic vascular resistance including the use of vasoconstrictors, and significant arterial calcification. A central venous line or pulmonary arterial catheterization may be considered in patients with refractory HF or with uncertain LV filling pressure [31]. A systolic BP > 90 to 100 mmHg should be targeted in most cases and treatment should be guided by clinical assessment for signs and symptoms of poor tissue perfusion, such as cold extremities, decreased urine output, and altered mental status, as well as using specific BP values. Fetal heart rate should be monitored in antepartum cases to detect early stage fetal distress.
Intravenous administration of loop diuretics is often required to reduce cardiac preload and pulmonary edema. Caution should be taken because excess diuresis may cause uteroplacental hypoperfusion and fetal distress. Intravenous vasodilators such as nitroglycerin reduce cardiac preload and afterload, but vasodilators may cause hypotension and uteroplacental hypoperfusion. Nitroprusside is not recommended during pregnancy due to the risk of fetal thiocyanate toxicity. Inotropes improve LV contractility and end-organ perfusion at the expense of increasing LV afterload. Both dobutamine and milrinone can be used during pregnancy. Dobutamine acts at β-adrenergic receptors and is preferred in cases of low systolic BP; it causes sinus tachycardia and may cause myocardial ischemia and arrhythmias. Milrinone is a phosphodiesterase IIIa inhibitor, which elevates the concentration of cyclic AMP, increases inotropy in cardiomyocytes and induces vasorelaxation in vascular smooth muscle. Milrinone is appropriate for patients with systolic BP ≥ 90 mmHg and concurrent β-blocker treatment. However, concerns of increased risk of mortality have yet to be resolved. Therefore, inotropes are reserved for persistent hypoperfusion despite adequate cardiac filling pressure [31].
In severe cases refractory to full medical therapy, mechanical cardiovascular support such as intra-aortic balloon pump or extracorporeal membrane oxygenation (ECMO) may be needed. ECMO can serve as a bridge to recovery or cardiac transplantation. Deciding on cardiac transplantation is challenging, as LV may recover slowly even after 6 months postpartum in PPCM; a LV assist device may be a reasonable option [2]. Cardiac transplantation is eventually required in a small number of cases. There is concern that graft survival may be inferior after cardiac transplantation in PPCM patients, which may be related to higher sensitization and overactive immunity [32]. Nevertheless, cardiac transplantation is a reasonable option for patients with refractory HF who cannot wean from intravenous inotropic agents or mechanical LV supporting devices.
Delivery
Delivery should be managed in a high-risk care setting by a multidisciplinary team. Principles of management are similar to those for women with other advanced cardiac diseases in pregnancy. The timing and mode of delivery are chosen based on maternal hemodynamic stability, obstetric indications including fetal condition, and the woman’s or couple’s wishes. The woman’s cardiovascular benefit is the highest priority in making a decision [2]. Spontaneous vaginal delivery is preferred in hemodynamically stable women. The advantages include avoiding abdominal surgery, less blood loss, low risk of thromboembolism, and early recovery compared to Cesarean section. In addition, regional anesthesia does not cause LV depression. Close monitoring during the antepartum period and 24 hours after delivery is important because hemodynamic changes are significant during labor [33]. Heparin should be stopped when uterine contractions start or 24 hours before a planned Cesarean section. Pain management is critically important to minimize hemodynamic stress. Epidural anesthesia is preferred for vaginal delivery. Efforts should be made to shorten the second stage of labor; assisted delivery by vacuum or forceps is recommended. A single dose of intramuscular oxytocin can facilitate optimal uterine contraction and reduce postpartum bleeding [2]. In the third stage of labor, autotransfusion of blood from the contracted uterus increases cardiac preload, which may cause cardiac decompensation in PPCM patients. Intravenous furosemide may be needed to prevent pulmonary congestion.
A planned Cesarean section is preferred in hemodynamically unstable or critically ill women. It facilitates intensive care including intravenous inotropes, mechanical ventilation, or continuous invasive monitoring [2,34]. A combination of spinal and epidural anesthesia is preferred but general anesthesia is often needed. Labor-associated hemodynamic changes can be attenuated during a Cesarean section, as compared to spontaneous vaginal delivery.
In general, the termination of pregnancy is not indicated in PPCM. However, urgent delivery irrespective of gestational age may be considered in patients with hemodynamic instability [2]. Termination of pregnancy is an important treatment for PPCM, and it enables effective medical treatment.
PROGNOSIS AND FOLLOW-UP
Prognosis is highly variable from complete recovery to cardiac death. LV function recovers completely or partially in the majority of cases. Full recovery of normal LV systolic function, often defined as LV EF > 50%, was reported in 23% to 72% of PPCM patients [35-37]. Recovery often occurs within 2 to 6 months after diagnosis but may be delayed for up to 5 years [38]. There is no published research concerning the duration of medical treatment in PPCM patients. In patients with LV systolic dysfunction, standard medical treatment for HF including β-blockers and ACEIs/ARBs should be continued for at least 12 months or more as some patients may experience delayed recovery [2,3]. Even in patients with normalized LV function, medical treatment is strongly recommended for at least 6 months after full recovery, because full recovery of LV size and EF does not necessarily imply long-term stability [3].
Despite maximal medical treatment, 20% to 25% of patients progress to end-stage HF over time. Cardiac transplantation or LV assist device treatment is performed in 4% to 11% of PPCM patients [2]. The reported maternal mortality was 3.3% to 30% over a period of greater than 6 months [12,38-43]. The majority of deaths occur within the first 3 to 6 months after diagnosis [44]. In recent studies, there has been a trend of decreasing mortality rates, probably owing to early diagnosis and improved HF management. However, PPCM mortality remains still high in young, previously healthy women. Major causes of death include progression to refractive HF, ventricular arrhythmia, and thromboembolism. Approximately one-third of survivors suffer from neurologic sequelae following cardiac arrest or stroke [45]. Independent factors predicting long-term prognosis are unclear; poor functional status, LV end-diastolic diameter ≥ 60 mm, LV EF ≤ 25% to 35%, and non-European ethnicity have been suggested [2,36,37]. LV EF is the most intensively studied parameter. Women with lower EF are most likely to experience serious clinical events such as death, cardiac transplantation, and thromboembolic complication [36]. However, complete recovery of LV is frequent, even in women with very severe LV dysfunction. The initial severity of LV dysfunction cannot be used to exclude treatments such as mechanical circulatory support in PPCM.
COUNSELING FOR SUBSEQUENT PREGNANCY
Subsequent pregnancy is an important issue in women with a history of PPCM. Subsequent pregnancy carries a 30% to 50% risk of recurrent PPCM [41,46]. LV systolic function tends to deteriorate during subsequent pregnancies in most women with a history of PPCM [2,41]. Termination of pregnancy may not prevent the onset of PPCM [2]. Based on probability, the risk associated with subsequent pregnancy might be stratified around LV function [47]. The risk of recurrent PPCM is higher in women with persistent LV dysfunction. Morbidity and mortality also tend to be higher in those with severe LV dysfunction [41,47]. Normalized LV EF does not guarantee a normal outcome in subsequent pregnancy. Therefore, all women with a history of PPCM should be informed that subsequent pregnancies entail significant risk for recurrent PPCM and even death. The current consensus advises strongly against subsequent pregnancy for all women experiencing PPCM, particularly for those who do not have a fully recovered LV systolic or diastolic function [2]. Full information concerning contraceptive options should be provided. Contraception using intrauterine devices is preferred because they are safe and effective. The estrogen-based oral pill is not recommended because of the risk of thromboembolism. Sterilization can be considered.
In women who are already pregnant, the decision of the woman and her family should be a priority. Because many women with PPCM remain healthy during subsequent pregnancies, particularly those with preserved LV systolic function, termination of pregnancy is not routinely indicated [48]. An individualized care plan is needed for women with a strong desire to maintain their pregnancy. In women with a history of PPCM, severe cardiovascular events may occur during late pregnancy or postpartum [47]. As LV dysfunction tends to deteriorate during late pregnancy, frequent echocardiographic monitoring (i.e., every 1 to 2 weeks) is helpful during the last trimester. Medical treatment of HF can prevent critical complications. β-Blockers with or without diuretics are the mainstay of treatment during the antepartum period [3]. After elective Cesarean section at week 36, full HF treatment including ACEIs/ARBs is available, and bromocriptine might be added.
CONCLUSIONS
PPCM threatens the health of pregnant women. Clinical suspicion of PPCM is critically important for early diagnosis. Echocardiography is valuable for screening, diagnosis, and follow-up after treatment. Standard HF treatment is recommended for PPCM patients; however, therapy should be adjusted considering fetal safety during pregnancy. Further studies are warranted to determine the pathophysiologic mechanisms, disease-specific biomarkers, effective treatments, and measures of disease-prevention for PPCM.
Notes
No potential conflict of interest relevant to this article was reported.