Fragmented QRS and abnormal creatine kinase-MB are predictors of coronary artery disease in patients with angina and normal electrocardiographys
Article information
Abstract
Background/Aims
Patients with symptoms of coronary artery disease (CAD) often display normal tracings or only nonspecific changes on electrocardiography (ECG). The aim of this study was to explore strategic elements of the ECG and other potential factors that are predictive of CAD in this scenario.
Methods
This was an observational study of 142 patients with the chief complaint of chest pain, each of whom presented with a normal ECG and was subjected to emergency coronary angiography (CAG). Two population subsets were identified: those patients (n = 97) with no significant stenotic lesions and those (n = 45) with the significant stenotic lesions of CAD.
Results
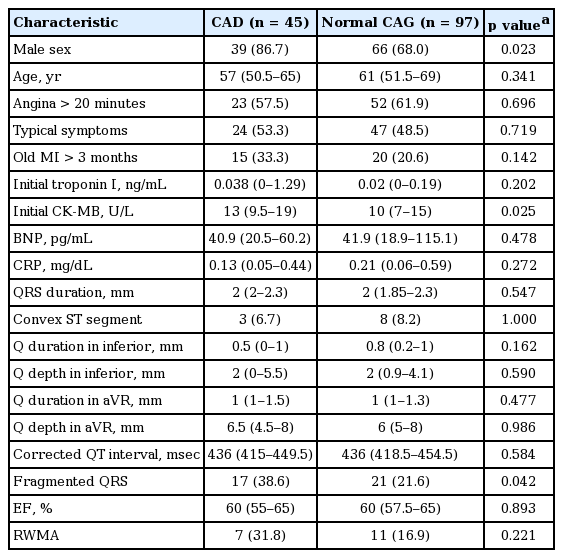
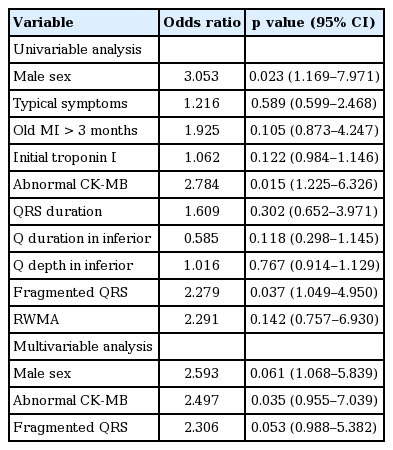
Those patients with normal or nonspecific ECGs and CAD (15.8%) were more likely to have left circumflex artery involvement (20% vs. 7%). In patients with normal ECGs and CAD (vs. normal CAG), male sex (86.7% vs. 68%, p = 0.023), creatine kinase-MB (CK-MB) levels > 10 U/L (13 vs. 10, p = 0.025), and fragmented QRS (fQRS) (38.6% vs. 21.6%, p = 0.042) occurred with greater frequency. In multivariable analysis, the following variables were significant predictors of CAD, given a normal ECG: male sex (odds ratio [OR], 2.593; 95% confidence interval [CI], 1.068 to 5.839); CK-MB (OR, 2.497; 95% CI, 0.955 to 7.039); and W- or M-shaped QRS complex (OR, 2.306; 95% CI 0.988 to 5.382).
Conclusions
In our view, male sex, elevated CK-MB (> 10 U/L), and fQRS complexes are suspects for CAD in patients with angina and unremarkable ECGs and should be considered screening tests.
INTRODUCTION
The electrocardiography (ECG) is pivotal in evaluating patients with suspected coronary artery disease (CAD). However, in patients who present to the emergency department (ED) with a chief complaint of chest pain, the initial ECG may be normal despite later discovery of CAD (Fig. 1). The rate at which this phenomenon occurs (3% to 16%) varies widely in the literature [1,2].
Patients who present to the ED with chest pain and with normal or nonspecific ECG interpretations have low rates of mortality and cardiac complications [3] and thus are considered low-risk [4]. Nevertheless, a normal or nonspecific ECG tracing does not exempt a patient from risk for cardiovascular events. Normal ECGs may contribute to increased mortality in patients who present to the ED who consequently are not hospitalized for acute cardiac ischemia [5]. There is also the possibility that changes consistent with myocardial ischemia may develop later, despite initial ECG tracings that are normal [6]. In one large study of patients with initially normal or nonspecific ECGs, respective hospital mortality rates of 5.7% and 8.7% were recorded [7].
To date, no algorithm exists to reliably predict adverse cardiovascular outcomes in patients with potential CAD based on a normal ECG alone (i.e., in the absence of ischemic changes) [8] or in conjunction with cardiac markers [9] or a history of angina [10]. Emergency echocardiography is a promising diagnostic tool, but it too has limitations. For example, regional wall motion abnormality (RWMA) was found in 22% of patients without CAD, and 12% of patients with CAD showed no evidence of RWMA [11].
In patients with normal or nonspecific ECGs, a means of differentiating those with and without CAD is clearly needed. The objective of our study was to determine the incidence of CAD in patients with normal or nonspecific ECGs, evaluating specific ECG features and other clinical parameters that are potentially predictive of CAD in this context.
METHODS
Study design
A retrospective observational study was conducted that consisted of reviewing all hospital admissions where patients presented to our ED with chest pain or discomfort that was suspicious of CAD, had ‘normal’ or ’nonspecific’ initial ECG interpretations, and had undergone emergency coronary angiography (CAG) based upon clinical assessments, including a history of recurrent or ongoing angina symptoms, elevated cardiac biomarkers, previous history or scores with high risks, and echocardiographic findings of heart dysfunction. The proportions of patients with verifiable significant stenotic lesions (of CAD) and those with no significant stenotic lesions were identified.
Study settings and population
This study was conducted at a high-end medical facility that functions as one of the regional cardiocerebrovascular centers in South Korea. Its ED serves an urban population of approximately 30,000 adult-patient visits per year. Data were compiled from 463 patients who consecutively presented between March 2010 and June 2014 and met all criteria as follows: (1) non-traumatic chest pain or discomfort that was suspicious of CAD as the chief complaint; (2) normal or nonspecific ECG interpretation, with no arrhythmia or ischemic change; and (3) CAD screening via CAG.
Study protocol
All patients who had undergone CAG were entered into a so-called ‘normal-ECG’ registry. Registry data included baseline characteristics, clinical presentation, and outcomes of diagnostic testing (i.e., cardiac biomarkers, ECG, and echocardiography). Subjects in the normal-ECG registry were stratified into two groups based on CAG results: patients who presented with normal CAGs (n = 97) and those who presented with abnormal CAGs (n = 45) involving a minimum of 70% stenosis in at least one vessel.
The clinical characteristics that were assessed were age, sex, duration of chest pain, typical chest pain, and known CAD. ‘Typical chest pain’ was equated with a sensation of squeezing and pressure (or heaviness). Pleuritic, positional, and prickly (or sharp) chest pain were excluded [10,12].
Patient eligibility was stipulated by an initial ECG that had been interpreted as ‘normal’ or ‘nonspecific’ according to Standardized Reporting Guidelines [13]. ECGs that read as ‘abnormal but not diagnostic of ischemia’ were also acceptable, with the following exclusions: bundle branch block, left ventricular (LV) hypertrophy with strain, minimal ST-T change in two or more contiguous leads, and pacemaker rhythm. Investigation of normal ECGs focused on duration of QRS complex, Q-wave characteristics, convexity of ST segment, corrected QT interval, and fragmented QRS (fQRS). An fQRS is defined by the presence of an additional R wave (R’) or notching in the nadir of the S wave, or the presence of > 1 R’ (fragmentation) in two contiguous leads with or without a typical bundle-branch block on a 12-lead ECG. Mean value, as the average of the three inferior leads, was used for estimating the duration of the QRS complex or Q wave.
Emergency echocardiographic screenings for CAD were also reviewed. Patients with echocardiographic evidence of RWMA prior to CAG were allowed, although changes (RWMA, ejection fraction) in echocardiographs that were done after the CAG were grounds for exclusion given the potential impact of cardiac catheterization on the motion of the cardiac wall.
Data analysis
To evaluate the relationships between risk factors and CAD in subjects in the normal-ECG registry, all categorical independent variables with more than two values were analyzed using Fisher exact test, and the Mann-Whitney U test was applied to all continuous independent variables. The significance of these relationships was repeatedly tested through univariable and multivariable analysis by binary logistic regression analysis. All calculations relied on standard software SPSS version 21 (IBM Co., Armonk, NY, USA), with statistical significance set at p < 0.05.
RESULTS
Incidence of patients with normal or nonspecific ECG interpretations
Of the 463 patients who had been admitted with chest pain or discomfort and subjected to CAG, initial ECGs (performed in our ED) were interpreted as normal or nonspecific in 142 cases. In addition, 286 of these 463 patients were diagnosed with CAD, including 45 of the 142 patients with normal or nonspecific ECG readings. The rate of normal or nonspecific ECG interpretations among patients with CAD was 15.8%.
Results of coronary angiography
CAD was defined as a 70% or more narrowing of the luminal diameter of the coronary artery by CAG. CAG was performed on all 463 patients who had accrued during the 3.25-year study timeframe, and in 286 of these patients, significant stenotic lesions were documented as single-vessel (left anterior descending artery [LAD, 29%], right coronary artery [RCA, 19%], or left circumflex artery [LCX, 7%]), or double-vessel (28%) or triple-vessel/left main (17%) CAD. In the 45 patients with normal or nonspecific ECGs and significant stenotic lesions, single-vessel disease predominated (LAD, 24%; RCA, 24%; LCX, 20%), with fewer instances of double-vessel (27%) or triple-vessel/left main (13%) disease; LCX lesions were also observed more frequently (20% vs. 7%) than in the all-inclusive group with CAD unrestricted by ECG.
Differentiating patients with normal or nonspecific ECGs by CAG group (CAD vs. normal)
Patients with CAD were more apt to be male (86.7% vs. 68%, p = 0.023), with notching of the QRS complex (fQRS) on ECG (38.6% vs. 21.6%, p = 0.042), compared with patients of normal status (Table 1). However, persistent chest pain (57.5% vs. 61.9%, p = 0.696) and chronic ischemic injury caused by previous old myocardial infarction (MI) (33.3% vs. 20.6%, p = 0.142) did not differ significantly by group.
Initial troponin I levels of patients with CAD exceeded those of patients with normal CAGs, although not to a statistically significant extent (0.038 ng/mL vs. 0.02 ng/mL, p = 0.202). In contrast, creatine kinase-MB (CK-MB) levels showed a positive correlation with acute coronary lesions (13 U/L vs. 10 U/L, p = 0.025). At a threshold > 10 U/L defined by the abnormal criteria of the biochemical test in our hospital (sensitivity, 75.6%; specificity, 47.3%), the accuracy of CK-MB in discriminating patients with significant stenotic lesions from normal counterparts was 0.621 (95% confidence interval [CI], 0.534 to 0.704), as estimated by the area under the receiver operating characteristic curve (Fig. 2).

Receiver operating characteristic curve showing discriminatory capability of creatine kinase-MB > 10 U/L. Area under curve (i.e., accuracy) is 0.621 (95% confidence interval, 0.534 to 0.704).
Pathologic Q waves in the inferior lead (0.5 mm vs. 0.8 mm, p = 0.162), changes in the Q wave in the aVR lead (1 mm vs. 1 mm, p = 0.477), and prolongation of QRS duration (2 mm vs. 2 mm, p = 0.547) were not distinctive in patients with CAD. Moreover, the impact of convex or concave ST-segments by group was uncertain (6.7% vs. 8.2%, p = 1.000), and corrected QT intervals did not differ significantly by group (436 msec vs. 436 msec, p = 0.584).
Within the subset of patients who had undergone emergency echocardiography prior to CAG, RWMA was rigorously investigated with respect to CAD, but it did not differ significantly by group (31.8% vs. 16.9%, p = 0.221).
In multivariable models, the odds ratios (ORs) for each variable as follows reflected significant group differences: males (OR, 2.593; 95% CI, 1.068 to 5.839); abnormal CK-MB (OR, 2.497; 95% CI, 0.955 to 7.039); and fQRS (OR, 2.306; 95% CI, 0.988 to 5.382) (Table 2). Hence, these parameters constituted significant predictors of CAD in our patients with angina and normal ECGs. Additionally, although we examined whether male, fQRS and abnormal CK-MB were related to degree of stenosis, no statistical association was observed (p = 0.372, p = 0.691, and p = 0.175, respectively).
Univariate analysis of factors related to coronary artery disease in patients with angina and normal electrocardiographys
The ECG of one such patient is illustrated in Fig. 1. This particular male showed fQRS in two leads (III and aVF), with CK-MB level (17 U/L) above the threshold and troponin I level at 0.006 ng/mL. CAG confirmed stenosis of the proximal LCX (80%), mid LAD (80%), and mid RCA (100%).
DISCUSSION
The ECG is a valid means of risk stratification for patients who present on an emergency basis with chest pain or discomfort. Studies indicate that low-risk patients with chest pain may be identified upon presentation at the ED by clinical evaluation plus ECG [14], and those patients with normal ECGs are considered low-risk [15,16]. However, clinically significant (albeit lower) short-term mortality rates have been recorded in patients with CAD and normal or nonspecific ECG tracings, compared with similar patients whose ECGs are abnormal [7], and a normal ECG has been cited as a significant factor in the failure to diagnose acute MI at emergency facilities [5]. Thus, the broad generalization that normal ECGs are low-risk may limit detection of ischemia in the following circumstances: (1) occlusive lesions of the LCX [17]; (2) prior acute MI; (3) established CAD [18]; (4) adequate collateral coronary circulation [19]; (5) transiently normal ECG [20]; and (6) small infarctions [17,21].
Our study illustrates that in patients with CAD, those with normal or nonspecific ECGs had a higher frequency of lesions involving the LCX. Chua et al. [17] similarly demonstrated that LCX-related acute MI that presented without ST-T changes has been underdiagnosed in ED settings, with ST-T changes absent in 23% of patients who were suffering LCX occlusion. In contrast, Caceres et al. [2] found that the frequency of LCX involvement in patients did not differ by ECG status (normal vs. abnormal).
Notching of QRS complexes has been the subject of much discussion in many recent studies. It is commonly upheld that fQRS correlates with an array of disorders, including CAD, dilated cardiomyopathy, cardiac sarcoidosis, arrhythmogenic right ventricular cardiomyopathy, Brugada syndrome, and long QT syndrome, as shown by myocardial perfusion-gated SPECT (single photon emission computed tomography) studies, CAG, and late gadolinium-enhanced cardiac magnetic resonance imaging [22-24]. In addition, fQRS has been identified as an independent prognosticator of mortality or cardiac events [25] in the setting of MI [23,26], with some evidence that equates the number of leads affected with the severity of the coronary lesions [27,28]. As demonstrated by Boineau [29], multiple MIs may lead to ECG modifications, with loss of Q waves and development of M- or W-shaped QRS complexes (fQRS). Other recent studies have shown that fQRS correlates poorly with myocardial scarring and is instead related to functional or anatomical ventricular abnormalities [30-32].
According to Lee and Goldman [33] the CK-MB level at the early onset of chest pain is a more accurate and sensitive index of MI than troponin I or troponin T. Wang et al. [21] discovered that among patients who presented with non-ST-segment elevation acute coronary syndromes, higher CK-MB (p < 0.0001) and troponin I (p = 0.001) levels corresponded with coronary arterial occlusion, which was documented by CAG in 27% (528/1,957) of their subjects.
We contend that male sex, abnormal CK-MB, and fQRS can be used as important diagnostic tools to predict coronary occlusion in patients with suspected CAD and normal ECGs. The association between CAD and a number of predictors (male, p = 0.696; fQRS, p = 0.053) in multivariable analysis was more decreased than that found in the univariable analysis. The reason the p value was higher than 0.05 in the multivariable analysis was the small sample size; better results will be shown through large multicenter studies. Herein, we evaluated fQRS complexes in normal patients without CAD, whereas most prior studies of fQRS have looked at high-risk diseases such as MI or cardiomyopathy; few researchers have focused on the significance of fQRS complexes in the general population.
Although a number of variables in our collected data generated statistically negative outcomes, the significance of these variables is still debatable. A Q wave is generally abnormal if its duration is 0.04 seconds or longer in lead I, in all three inferior leads (II, III, aVF), or in leads V3 through V6 [34], and a pathologic Q wave is one involving two or more contiguous leads. The presence of a Q wave in the aftermath of MI is associated with higher in-hospital morbidity and mortality [35]. Furthermore, a history of prior MI in patients with non-ST-elevation CAD poses a significant risk [21]. Some sources have shown that prolonged corrected QT intervals are predictive of cardiac events after MI [36]. Elsewhere, however, pathologic Q waves, corrected QT intervals, and previous ischemic heart disease were unrelated to prognosis (i.e., major adverse cardiac events) [37]. Still, one publication does maintain that fQRS complexes are superior to pathologic Q waves in terms of their sensitivity to and negative predictive value for myocardial scarring [38]. We similarly contend that fQRS is a superior index of CAD to pathologic Q waves or history of prior MI.
In our hands, RWMA detected by emergency echocardiography (done prior to CAG and performed by a cardiologist or sonographer) curiously showed no statistical association with significant stenotic lesions of a coronary artery. Possible explanations are as follows: (1) latent stress-related cardiomyopathy, such as isolated basal LV dysfunction, global LV hypokinesia, and other wall motion abnormalities in non-coronary distribution (aside from Takotsubo cardiomyopathy) [39], in patients with angina and normal ECGs; (2) residual RWMA as from previous MI; (3) RWMA undetected because of minute or diffuse multiple coronary lesions; (4) execution error by untrained cardiologists or sonographers; and (5) paucity of suitable cases.
This study has a number of acknowledged limitations, one being the limited (for statistical purposes) number of patients with normal ECGs who were admitted for angina through our emergency services. Another is the retrospective nature of the study and its restriction to a single institution. Nevertheless, nearly all required data were collected successfully with the exception of some laboratory results. Finally, collateral coronary arterial circulation was not investigated owing to the incompleteness of our records, and patients (with potential CAD) who did not undergo CAG were excluded from study.
In conclusion, our data indicate that male sex, CK-MB levels beyond a set threshold, and fQRS complexes on ECG are predictive of CAD in patients with angina and normal ECGs. The presence of fQRS on ECG and elevated CK-MB in this context also appear to reflect coronary arterial stenosis in 70% or more of cases. These findings clearly merit further study.
KEY MESSAGE
1. Fragmented QRS (fQRS) related with scar or left ventricular dysfunction was observed in patients with structural heart disease as well as with normal hearts.
2. A fQRS was shown in 10% to 16% patients who had been analyzed as having normal sinus rhythm and certified coronary artery lesions by coronary angiography (CAG).
3. Patients with coronary artery disease were more apt to be male (86.7% vs. 68%, p = 0.023) with notching of the QRS complex (fQRS) on electrocardiography (38.6% vs. 21.6%, p = 0.042) compared with patients who showed normal CAG.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by the Dong-A University Research Fund.