Loeffler endocarditis in chronic eosinophilic leukemia with FIP1L1/PDGFRA rearrangement: full recovery with low dose imatinib
Article information
To the Editor,
Chronic eosinophilic leukemia (CEL) with FIP1L1/PDGFRA (factor interacting with PAPOLA and CPSF1/platelet-derived growth factor receptor α) rearrangement is a rare cause of hypereosinophilia, with few reports in Korea [1]. One of the most critical types of organ damage caused by infiltration with eosinophils is Loeffler endocarditis, with fibrous thickening of the endocardium leading to apical obliteration and restrictive cardiomyopathy. There are some reports of Loeffler endocarditis in patients with hypereosinophilic syndrome [2,3]. This is the first reported case of CEL with FIP1L1/PDGFRA rearrangement combined with Loeffler endocarditis in Korea.
A 28-year-old man presented to the emergency department with a headache, dyspnea, and aphasia. Acute infarction in the left posterior middle cerebral artery territory was found on brain magnetic resonance imaging. A transthoracic echocardiogram revealed endocardial thickening and thrombus in both ventricular apices, typical finding of Loeffler endocarditis (Fig. 1A). Initial laboratory findings were as follows: hemoglobin 12.5 g/dL, white blood cell (WBC) count 26,400/µL (eosinophils 12,302/µL), and platelet count 44,000/µL. All antibody tests for parasites were negative and no other allergic symptoms were present. In the bone marrow (BM) aspirate, marked eosinophilia, often with bizarre nuclear lobulation, and dysgranulation were found (Fig. 2A). The biopsy specimen showed hypercellular (90%) marrow for age (Fig. 2B). Fluorescence in situ hybridization (FISH) analysis was positive (92%) for FIP1L1/PDGFRA fusion (Fig. 3A). According to the 2008 World Health Organization classification, he was diagnosed with myeloid and lymphoid neoplasm (CEL) associated with FIP1L1/PDGFRA. Low-dose imatinib (100 mg) and anticoagulation with enoxaparin were started. The WBC, eosinophil and platelet count normalized within 10 days of imatinib treatment. After 2 months of treatment, FISH for FIP1L1/PDGFRA fusion became negative (Fig. 3B). Endocardial thickening and thrombus in both ventricles resolved on serial echocardiography during 3 months of treatment with imatinib (Fig. 1B and 1C). Follow-up BM aspiration and biopsy slides after 3 months of treatment showed normocellular marrow with 1.3% of eosinophil count (Fig. 2C and 2D). The patient remains in complete cytogenetic remission and had no signs or symptoms for over a year.

Serial transthoracic echocardiogram (TTE) in apical 4-chamber view. (A) Initial TTE images demonstrate large echodense masses attached to significantly thickened biventricular endocardial walls (white arrows). (B) After 15 days of treatment (warfarin and imatinib), TTE demonstrated complete regression of echogenic wall thickening in the left ventricular apex, but was still noted in the right ventricular apex (arrowheads). (C) Follow-up TTE was performed after 3 months of treatment, with no further evidence of wall thickening or thrombi in both ventricular apices.
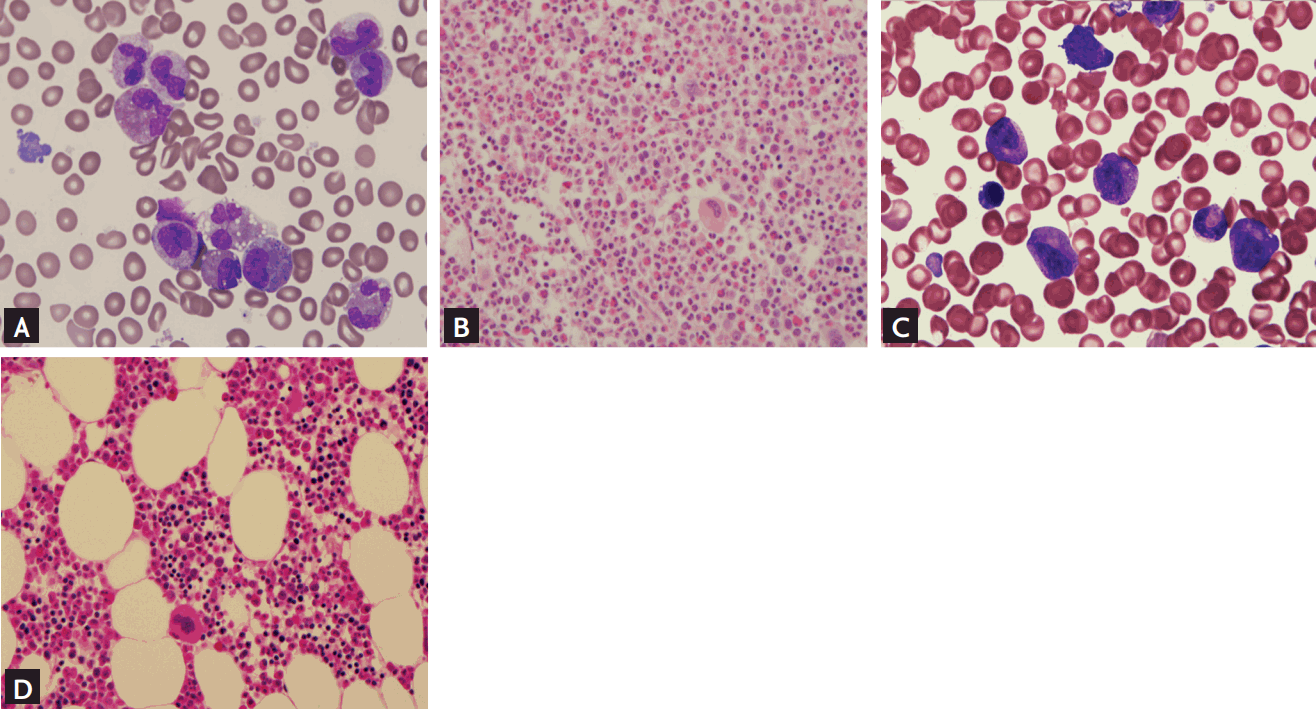
Bone marrow (BM) aspirate and biopsy findings. (A) BM aspiration slide at baseline (×1,000). Myeloid:erythroid ratio was 13.7:1. Granulocytic precursors were increased. Eosinophils accounted for up to 28.0% of the marrow absolute neutrophil count. Abnormal features of eosinophils were sparse granulation with clear areas of cytoplasm, cytoplasmic vacuolation, bizarre nuclear lobulation, and nuclear hypersegmentation. (B) BM slide at baseline (H&E, ×400). The biopsy specimen showed hypercellular (90%) marrow for age. Megakaryocytes were increased and nucleated cells were mostly eosinophils and other hematopoietic cells. (C) BM aspiration slide after 3 months of treatment (×1,000). Eosinophils with normal morphology were counted up to 1.3% of marrow neutrophil. (D) BM slide after 3 months of treatment showed normocellular (30% to 40%) marrow for his age (H&E, ×400).
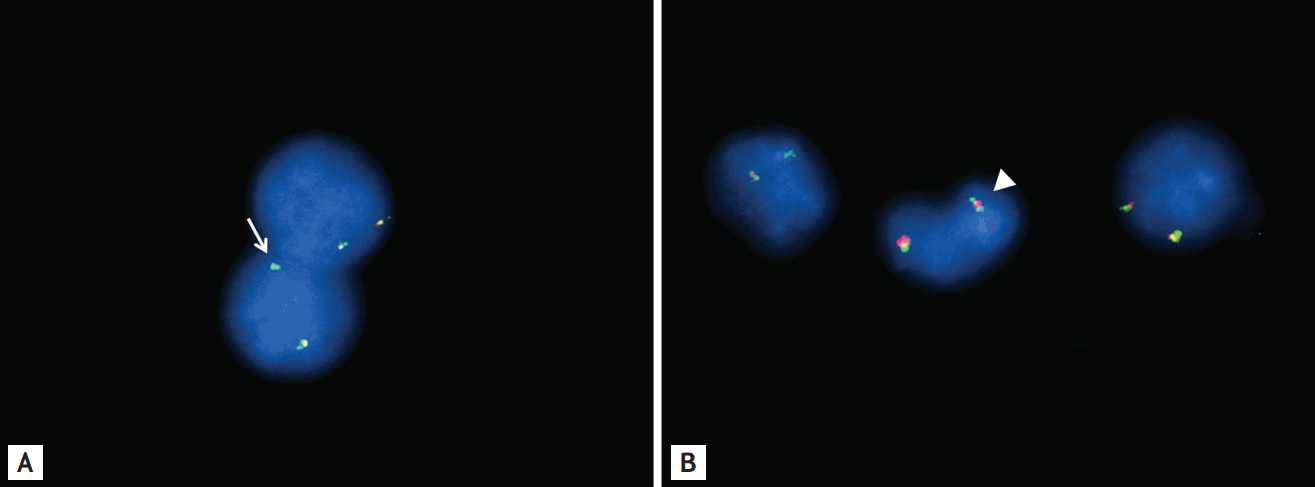
Fluorescence in situ hybridization (FISH) findings for FIP1L1/PDGFRA (factor interacting with PAPOLA and CPSF1/ platelet-derived growth factor receptor α) fusion. (A) FISH result at diagnosis shows abnormal green-green fusion signal lacking an orange signal (white arrow). (B) FISH result after 2 months of treatment. All cells show normal tri-color signal with green, orange, and green (arrowhead).
CEL with FIP1L1/PDGFRA rearrangement is very rare, and only four cases have been reported in Korea [1]. Two had cardiac findings with valvular regurgitation. Cardiac involvement is frequently reported in patients with hypereosinophilia. Among these, patients with restrictive cardiomyopathy known as Loeffler endocarditis have a very poor prognosis [2,3]. The prognosis of Loeffler endocarditis depends on how well the eosinophilia is controlled [4]. This is the first report of CEL with Loeffler endocarditis in Korea. CEL with FIP1L1/PDGFRA showed dramatic response to imatinib mesylate, unlike other causes of hypereosinophilia [5]. In the present case, hematologic and cytogenetic remission was achieved with imatinib 100 mg daily, and cardiac involvement also improved. Although neurologic deficits due to multiple cerebral infarctions and severe endocardial thickening and thrombi were observed on admission, these abnormalities completely resolved with low-dose imatinib and anticoagulation. Recurrence of thrombus or infarction was not observed after discontinuation of anticoagulation when cytogenetic remission was confirmed. In conclusion, cardiac dysfunction in patients with hypereosinophilia can be fatal, but a targeted agent such as imatinib showed dramatic cardiac improvement in a CEL patient with FIP1L1/PDGFRA rearrangement. Thus, early workup and active management for eosinophilia should be performed in cases of cardiac dysfunction combined with hypereosinophilia.
Notes
No potential conflict of interest relevant to this article was reported.