New insights into the role of renal resident cells in the pathogenesis of lupus nephritis
Article information
Abstract
Systemic lupus erythematosus (SLE), an autoimmune disease of unknown etiology, is characterized by the production of autoantibodies and end-organ damage. Lupus nephritis affects up to 70% of patients with SLE and is the most critical predictor of morbidity and mortality. The immunopathogenesis of SLE is complex and most clinical trials of biologics targeting immune cells or their mediators have failed to show efficacy in SLE patients. It has therefore become increasingly clear that additional, local factors give rise to the inflammation and organ damage. In this review, we describe recent advances in the role of renal resident cells, including podocytes, mesangial cells, and epithelial cells, in the pathogenesis of lupus nephritis.
INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disease that affects multiple organs and has significant mortality. Its etiology is multifactorial and includes both environmental and genetic influences. Despite considerable progress in research into the pathogenesis of SLE, through the use of genetic variant identification, murine models, gene expression studies, and epigenetic analysis, the exact pathogenesis of SLE remains poorly understood [1,2]. Nevertheless, contributions of various immunologic abnormalities to the pathogenesis of SLE have been confirmed, including the defective clearance of apoptotic cells, a loss of tolerance to self-antigens, aberrant activation of T- and B-cells, altered cytokine profiles, such as those of type I interferon (IFN), the involvement of neutrophilic extracellular traps, and pathogenic autoantibody production [3,4]. The serologic hallmark of SLE is the production of autoantibodies, which are mostly directed against nuclear antigens, including double-stranded DNA (dsDNA), small nuclear ribonucleoproteins, and nucleosomes, but also against antigens located in the cytoplasm, on the cell surface, and secreted by the cell [1,5].
Renal involvement, characterized by immune complex deposition, inflammation, and scarring of the glomeruli and interstitium, is the most common and severe clinical manifestation of SLE. However, only a few autoantibodies have been found to specifically contribute to the disease-related injury seen in mice and humans, including anti-blood cell antibodies causing cytopenia, anti-dsDNA antibodies causing nephritis, and anti-phospholipid antibodies causing fetal distress [6]. From a clinical perspective, most trials of biologics targeting immune cells have failed to show efficacy in SLE patients. For example, rituximab, a genetically engineered chimeric monoclonal IgG1-κ antibody targeting CD20 on B-cells, was expected to show efficacy in SLE patients because it directly targets B-cells, which are responsible for autoantibody production. However, two large phase III clinical trials did not verify the clinical efficacy of rituximab in patients with non-renal lupus and lupus nephritis [7,8]. In addition, the use of congenic mice has shown that distinct chromosomal regions determine the development of autoimmunity and chronic kidney damage [9]. Another study demonstrated that SLE patients in clinical remission continue to produce elevated levels of self-reactive and polyreactive antibodies [10]. These findings suggest that autoimmunity and tissue damage are independent processes. Thus, to achieve a comprehensive understanding of the pathogenesis of lupus, greater attention will need to be paid to tissue resident cells and the tissue-specific factors involved in the tissue damage process.
Recent evidence supports a direct role for renal resident cells, including podocytes, mesangial cells, and tubular epithelial cells, in the development of lupus nephritis. In this review, we provide an overview of the role of renal resident cells, and especially podocytes, in the pathogenesis of lupus nephritis.
THE ROLE OF PODOCYTES
Podocytes are highly specialized cells that reside on the visceral side of Bowman’s capsule and wrap around glomerular capillaries. They are essential components of the glomerular filtration apparatus and are critical for the maintenance of renal function [11]. By expressing markers such as synaptopodin, nephrin, podocin, and Wilms’ tumor protein, podocytes play an important role in maintaining the integrity of the glomerular filtration barrier. Animal and human studies have demonstrated a strong correlation between defects in podocyte anchoring and several glomerular abnormalities, verifying the role of podocytes in the onset of renal diseases. In addition, extensive human genetic studies have indicated that monogenic mutations in podocyte proteins are associated with specific renal pathologic phenotypes. This capacity of podocyte injury or loss to initiate glomerular damage may be related to the development of chronic kidney disease [12]. Disruption of the glomerular filtration barrier allows passage of large molecules through the glomerulus, which, together with apoptotic debris, can activate tubular interstitial cells and resident dendritic cells to initiate the pathways leading to chronic inflammatory disease [13].
Immune cell-like features of glomerular podocytes
In addition to helping to maintain the glomerular filtration barrier, podocytes exhibit the properties of immune cells (Table 1) [14-25] and may be involved in adaptive immunity. For example, podocytes express CD80, which costimulates lymphocytes; mice lacking CD80 are protected from lipopolysaccharide (LPS)-induced nephrotic syndrome, suggesting a link between podocyte CD80 expression and proteinuria [14]. Podocytes also express major histocompatibility complex (MHC) class II [15] and neonatal Fc receptor, a receptor protein of antigen-presenting lymphocytes [16]. Goldwich et al. [26] reported that deleting MHC class II exclusively on podocytes prevents the induction of experimental anti-glomerular basement membrane nephritis. Podocytes ingest the soluble antigens that activate CD4+ T cells and cross-present exogenous antigen on MHC class I molecules to CD8+ T cells, which suggests that podocytes participate in the antigen-specific activation of adaptive immune responses [26].
Podocytes also express diverse genes required for the innate immune response, including those encoding the pattern recognition receptors (PRRs) that sense both pathogen-associated molecular patterns and damage-associated molecular patterns (DAMPs). Toll-like receptors (TLRs) are the most well-known PRRs. Isolated glomeruli express TLR1–TLR9 and TLR11 mRNA, with the highest levels of expression being those of TLR3 and TLR4 [17]. In vitro treatment of cells with TLR4 ligands, such as LPS and fibrinogen, promotes a marked induction of chemokines [17]. The receptor for advanced glycation endproducts (RAGE) is a PRR involved in several innate immune responses. RAGE can use DAMPs, including advanced glycation endproducts and high-mobility group box protein 1, as its ligands. RAGE is expressed in podocytes and up-regulated in both human and mouse glomerular diseases [18]. In a murine model of adriamycin-induced glomerulosclerosis, RAGE was shown to mediate podocyte injury [19].
In addition, podocytes produce the proinflammatory cytokine interleukin 23 (IL-23) [20], which is a potent stimulator of renal inflammation. IL-23 promotes the differentiation of T-cells into Th17 cells, γδ T-cells, and double-negative T-cells [27]. The resulting stimulation of neutrophil infiltration into the tubular interstitium leads to the establishment of chronic inflammation.
The role of podocytes in lupus nephritis
Proteinuria is the most important feature of lupus nephritis and its development is associated with podocyte dysfunction. Therefore, the development of lupus nephritis likely accompanies podocyte injury. Indeed, podocyte injury is common in patients with lupus nephritis, as shown in a large cohort study of patients with renal-biopsy-proven lupus nephritis [28].
Several lines of evidence support the role of podocytes in the pathogenesis of lupus nephritis [14,21-24,29,30]. Podocyte expression of costimulatory molecules, such as CD80, correlates with the severity of human lupus nephritis [14]. TLR9 is up-regulated within the glomeruli of patients with lupus nephritis, but not in normal kidneys [24]. Ichinose et al. [22] showed that immunoglobulin G (IgG) from lupus nephritis patients enters podocytes, where it up-regulates calcium/calmodulin-dependent protein kinase IV (CaMK4), which is followed by the increased expression of genes linked to podocyte damage and T-cell activation. This finding suggests the targeted inhibition of CaMK4 in podocytes as a therapeutic strategy in lupus nephritis [22].
The major component of inflammasomes, Nod-like receptor protein 3 (NLRP3), the apoptosis-associated protein ASC (apoptosis-associated speck-like protein containing a CARD), and caspase 1, are also expressed in podocytes [23]. NLRP3-expressing inflammasomes are activated in the podocytes of lupus-prone mice, and in those of patients with lupus nephritis. In the former, the inhibition of NLRP3 ameliorates proteinuria, renal histologic lesions, and podocyte foot process effacement [21], suggesting that NLRP3 activation is involved in the pathogenesis of podocyte injury and in the development of proteinuria in lupus nephritis.
THE ROLE OF MESANGIAL CELLS
Mesangial cells are specialized cells in the kidney that make up the mesangium of the glomerulus. Together with the mesangial matrix, they form the vascular pole of the renal corpuscle. The main function of mesangial cells is to remove trapped residues and aggregated protein from the basement membrane; thus, keeping it and the glomerular filtration apparatus free of debris [31].
Mesangial cells have also been implicated in the pathogenesis of lupus nephritis [32]. Studies have shown that mesangial cells express TLRs [33,34], and when stimulated with TLR-3 ligand they produce type I IFN [33], the major cytokine in the development of SLE. The production of type I IFN by renal resident cells, including mesangial cells, may aggravate autoimmune kidney diseases [33,35]. Anti-dsDNA antibodies are essential to the diagnosis of SLE, and their levels correlate with disease activity. Yung et al. [36,37] demonstrated that anti-dsDNA antibodies bind to mesangial cells and trigger downstream inflammatory and fibrotic pathways, including the protein kinase C (PKC) and mitogen-activated protein kinase (MAPK) signaling pathways, in addition to increasing both the secretion of proinflammatory cytokines and matrix protein deposition, all of which contribute to pathologic processes in the renal parenchyma.
Mesangial cells produce IL-6, and probably additional cytokines, which could independently contribute to glomerulonephritis. Mesangial cells lacking CaMK4 produce decreased amounts of IL-6 [38]. An early study showed that IL-6 on its own can drive the development of glomerulonephritis [39,40].
THE ROLE OF RENAL TUBULAR EPITHELIAL CELLS
Renal tubular epithelial cells are another type of renal resident cell likely to be involved in the pathophysiology of lupus nephritis. For example, renal tubular epithelial cells produce pathogenic cytokines, including type I IFN [41] and B-cell activating factor (BAFF) [42], both of which are very important to the development of SLE. BAFF expression in the kidney of lupus-prone mice correlates with disease activity. In biopsies of patients with diffuse proliferative lupus nephritis, BAFF expression on tubular epithelial cells correlates with the histopathological activity index [42]. In addition, renal tubular epithelial cells from patients with lupus nephritis express the co-stimulatory molecule B7-H4, suggesting the ability of these cells to activate T-cells [43].
The incubation of renal tubular epithelial cells with human anti-dsDNA antibodies leads to the sequential up-regulation of tumor necrosis factor α, IL-1β, and IL-6 [44], indicating a contribution by these cells to inflammatory processes in the tubulointerstitium in lupus nephritis.
CONCLUSIONS
Conventional therapy for SLE has been based on non-specific immunosuppressants, which have limited clinical efficacy and cause severe adverse events. As our understanding of the immunopathogenesis of SLE has progressed, new classes of drugs and biologics that target immune cells in patients with this disease have been developed. However, their clinical efficacy is unsatisfactory and none of the biologics has proven to be effective in the treatment of lupus nephritis, a common manifestation and critical determinant in the prognosis of SLE patients.
Given that autoimmunity can occur independently of tissue injury mechanisms, it is necessary to understand the process that alters resident cell function. Podocytes are an essential component of the glomerular filtration barrier and their dysfunction is directly connected with the initiation of glomerulonephritis. Therefore, the identification of a common intracellular pathway leading to both immune cell aberration and podocyte dysfunction will aid in the development of therapeutics targeting both immune cells and podocytes in a novel and effective therapeutic strategy (Fig. 1).
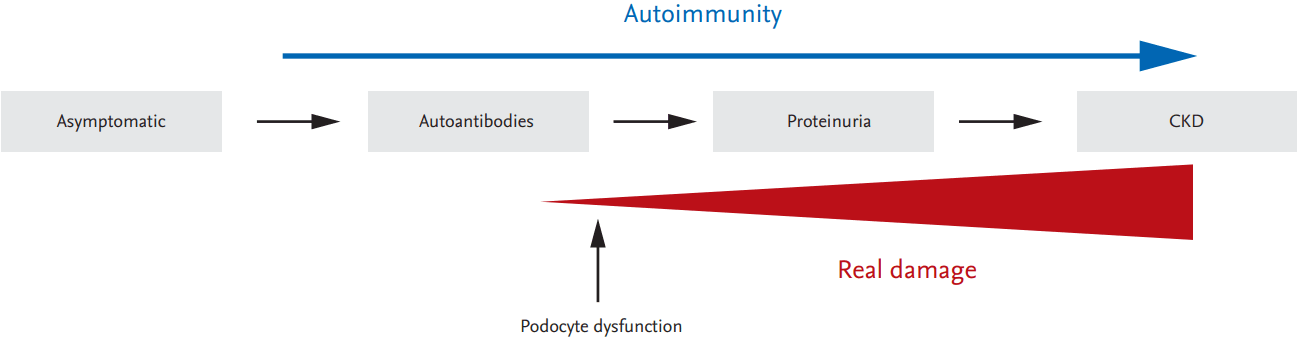
Proposed model of the development of lupus nephritis. Autoimmunity persists from the asymptomatic stage to the stage marked by chronic kidney disease (CKD). Podocyte dysfunction ,may already occur at an early stage of the kidney damage process, ultimately leading to overt proteinuria and CKD. Identification of a common intracellular pathway that mediates not only aberrant immune cell activation related to autoimmunity but also podocyte dysfunction, will lead to a better understanding of the pathogenesis of lupus nephritis.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, ICT and Future Planning (2015R1A2A2A01003886).
