Efficacy and safety of tocilizumab in Korean patients with active rheumatoid arthritis
Article information
Abstract
Background/Aims
To investigate the efficacy and safety of tocilizumab (TCZ) humanized anti-interleukin-6 receptor monoclonal antibody, in Korean patients with active rheumatoid arthritis (RA) refractory to conventional disease modifying anti-rheumatic drugs (DMARDs) including methotrexate (MTX)
Methods
The main study was a 24-week, randomized, double-blind, controlled trial that was followed by a 48-week, open-labeled, extension phase. TCZ (8 mg/kg) or placebo was intravenously administered every 4 weeks.
Results
Those treated with TCZ showed more favorable outcomes in terms of 20% according to the American College of Rheumatology response criteria (ACR20) and ACR50 responses, individual parameters of ACR core set, disease activity score in 28 joints (DAS28) remission, and European League Against Rheumatism (EULAR) response at week 24. These improvements were maintained or increased during the extension period. DAS28 remission at week 72 was associated with EULAR good response at week 12. The patients who experienced any adverse event (AE) were more frequent in the TCZ group compared to the placebo group. Most AEs were mild or moderate in intensity, although TCZ therapy had possible AEs including serious infection, abnormal liver function, and atherogenic lipid profile.
Conclusions
TCZ infusion add-on is highly efficacious and well-tolerated in Korean patients with active RA refractory to conventional DMARDs including MTX. EULAR good response at week 12 could predict DAS28 remission at week 72.
INTRODUCTION
Rheumatoid arthritis (RA) is a systemic inflammatory disease characterized by fatigue, joint pain, and swelling, which results in loss of function, progressive disability, and increased morbidity and mortality [1,2]. Targeting the proinflammatory cytokine tumor necrosis factor (TNF) using monoclonal antibody is an effective therapeutic option for RA patients, especially those with an inadequate response to conventional disease modifying anti-rheumatic drugs (DMARDs) including methotrexate (MTX) [2]. However, up to 40% of patients have an inadequate response to anti-TNF treatment and there has been an unmet need for new drugs with new modes of action for RA [3].
Interleukin-6 (IL-6) is a proinflammatory cytokine involved in multiple immunologic processes, such as T cell activation, B cell proliferation, and initiation of acutephase protein [4]. The serum level of IL-6 has been correlated with disease activity of RA. Thus, IL-6 is thought to play a major pathological role in RA [5]. Tocilizumab (TCZ) is a humanized anti-IL-6 receptor monoclonal antibody that can block IL-6 receptor signaling and the subsequent pro-inflammatory process [6].
Several clinical trials have demonstrated the efficacy and safety of TCZ monotherapy or in combination with conventional DMARDs in patients with moderate to severe RA [7-11]. Recently, the European League Against Rheumatism (EULAR) recommended TCZ as a biologic DMARD for patients with RA refractory to conventional DMARDs [12].
Most clinical trials for TCZ have been conducted in Caucasian or Japanese people. We investigated the efficacy and safety of TCZ in Korean patients with active RA refractory to conventional DMARDs including MTX. We also conducted a post hoc study to look for clinical parameters associated with disease activity score in 28 joints (DAS28) remission induced by TCZ treatment.
METHODS
Patients
Patients were eligible for the study if they had been diagnosed with RA according to the 1987 American College of Rheumatology (ACR) criteria [13] and had active disease refractory to MTX with or without other conventional DMARDs. Active RA was defined by a swollen joint count (SJC) ≥ 6 from the total of 66, tender joint count (TJC) ≥ 8 from the total of 68, and serum C-reactive protein level ≥ 1 mg/dL or erythrocyte sedimentation rate (ESR) ≥ 28 mm/hr. Stable dose of DMARDs was required for at least 8 weeks prior to study entry. Oral glucocorticoids (up to prednisone 10 mg/day equivalents) and non-steroidal anti-inflammatory drugs (NSAIDs) were permitted if the dose was stable for at least 6 weeks. Patients were excluded if they had concurrent active illnesses, such as infection and malignancy, previous history of hypersensitivity, or contraindication to treatment with human, humanized, or mouse monoclonal antibody. All subjects were screened for latent tuberculosis according to the Korean guideline for patients starting therapy with biologic agents. Those with latent tuberculosis were excluded if they refused the medication [14].
Study design
The study was composed of main and extension trials. The main study was a 24-week, phase III, randomized, double-blind, placebo-controlled, multicenter trial. It was conducted from October 2009 to October 2010. A total of 140 patients were screened and 99 patients were randomly assigned to either the TCZ group or placebo group. Randomization was stratified according to whether patients used MTX alone or in combination with other DMARDs. TCZ at a dose of 8 mg/kg or placebo was administered intravenously in a blinded manner with an approximately 60-minute infusion every 4 weeks. Background treatment with DMARDs was maintained during the study. If a patient showed no improvement by week 12 or less than 20% improvement by week 16, based on SJC and TJC, one rescue therapy was permitted. Rescue therapy included change of DMARDs, increase in dose of DMARDs, intra-articular glucocorticoid injection to one inflamed joint, or increase in dose of oral glucocorticoid (up to prednisone 10 mg/day equivalents). Those who received the rescue therapy were regarded as non-responders for ACR response.
Patients who completed the main study or withdrew from the main study due to lack of efficacy of study drug could be enrolled in a subsequent 48-week, open-labeled extension trial conducted to September 2011. All patients who participated in the extension study were given TCZ at the same dose and interval with the main study. Modification of NSAID, DMARD, or glucocorticoid therapy was allowed during the extension period according to the investigator’s decision. The dose of oral glucocorticoid could be increased (up to prednisone 10 mg/day equivalents) or decreased until week 40. Intra-articular glucocorticoid injection was also permitted until week 40.
Efficacy assessment
The primary endpoint of the main trial was the proportion of patients who achieved improvement of 20% according to the ACR response criteria (ACR20) [15] at week 24. Secondary end points included the proportion of patients with 50% and 70% improvement by ACR criteria (ACR50 and ACR70, respectively); remission based on DAS28 using ESR [16]; EULAR response [17]; the proportion of patients who withdrew from the trial due to lack of efficacy and received the rescue medication; change in each ACR core set parameter, DAS28 score, hemoglobin level, rheumatoid factor (RF) titer, and Health Assessment Questionnaire disability index (HAQ-DI) at week 24 [18]; and the time interval to reach ACR20, ACR50, and ACR70 responses. Efficacy variables were assessed as secondary endpoints in the extension study.
Safety assessment
Safety was assessed as secondary and primary endpoints of the main and the extension study, respectively. Adverse events (AEs), serious AEs, adverse drug reactions (ADRs), and clinically significant changes in laboratory test results during the study period were collected. All AE and ADR data were summarized according to World Health Organization Adverse Reaction Terminology system organ class and preferred terms [19].
Statistical analyses
The sample size for the main study was calculated with a significance level of 0.05 (two-sided test) and statistical power of 80% to verify a hypothetical difference of 30% at ACR20 response between the TCZ and the placebo groups with an intention-to-treat analysis. The clinical trial was designed with 45 subjects in each group considering the uncertainties of calculation and subject dropout during the study.
The analysis of efficacy was performed on an intention-to-treat basis in the main study. Primary and secondary outcome variables were compared between the TCZ and the placebo groups by chi-square test, Fisher exact test, two-sample t test, or Wilcoxon’s rank sum test. The last observation carried forward rule was applied for the missing SJC or TJC data. Safety analysis was conducted based on the number of AEs that occurred during the study. Chi-square test or Fisher exact test was used to compare the incidence of AEs. Paired t test was used to analyze the changes of the continuous variables. To search for parameters associated with DAS28 remission at week 72, univariate analysis was performed when comparing the patients with remission and those without remission. All statistical comparisons were done by two-tailed analyses and the difference was considered as significant if p value was < 0.05.
Ethics statement
The study was approved by relevant Institutional Review Boards (0904-037-278) and written informed consent was obtained from each patient. The study was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice (GCP) and the Declaration of Helsinki. These trials are registered at www.clinicaltrial.gov as NCT01211834 and NCT01256736.
RESULTS
Characteristics of the patients
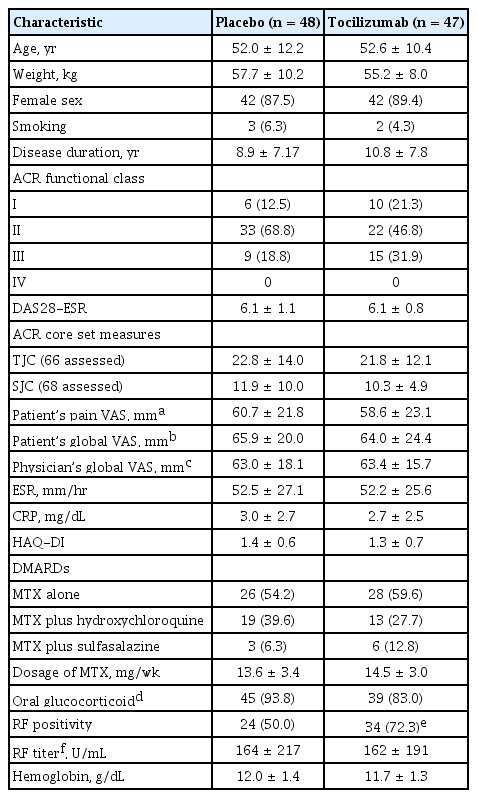
A total of 99 patients were enrolled in the main 24-week clinical trial from 11 institutions. They were randomized to the TCZ (n = 48) or the placebo group (n = 51). Eighty patients (80.8%) completed this study. Subsequent to the main study, these 80 completers and nine patients who withdrew from the main study due to inefficacy of the placebo (n = 5) and GCP or protocol violations (n = 4; three from the placebo group and one from the TCZ group) participated in the open-labeled extension study. Seventy-two (80%) completed the extension study (Fig. 1). Baseline characteristics of the TCZ and placebo groups from the main study were comparable, except for the proportion of patients with RF (Table 1).

Patient disposition. TCZ, tocilizumab; GCP, Good Clinical Practice; AE, adverse effect. a One patient was related with both AE and GCP violation.
Efficacy assessment
Main study
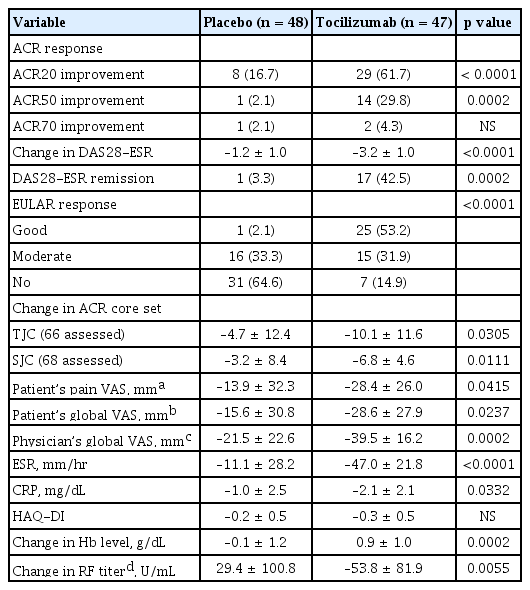
Patients treated with TCZ showed significant improvements by clinical and laboratory parameters compared with those treated with placebo (Table 2). The proportion of ACR20 responders was significantly higher in the TCZ group than in the placebo group (61.7% vs. 16.7%, p < 0.0001) at week 24. The proportion of patients with ACR50 response, DAS28 remission, and EULAR good response were also significantly increased in TCZ-treated patients. The TCZ-treated patients revealed favorable outcomes in change of DAS28 from the baseline and individual parameters of ACR core set, except for HAQ-DI. Efficacy of TCZ was apparent as early as 4 to 8 weeks after infusion. The level of hemoglobin was significantly elevated at week 24 in the TCZ group compared with the placebo group. The titer of RF significantly decreased in seropositive patients with TCZ treatment at week 24, whereas it increased in those with placebo (Table 2).
Extension study
Forty-two patients from the TCZ group and 47 patients from the placebo group participated in the extension study. The efficacy endpoints were assessed for the patients who were treated with TCZ successively during the main and extension study periods. At week 72, ACR20, ACR50, and ACR70 response was observed in 87.9%, 63.6%, and 36.4% of the patients, respectively. Eightyeight percent and 66.7% of the patients achieved EULAR good response and DAS28 remission at week 72, respectively (Table 3). Those who began to be infused with TCZ from the extension study caught up to the response of the patients who received TCZ from the main study (Fig. 2). The level of hemoglobin at week 72 significantly increased from baseline (p < 0.0001). The titer of RF at week 72 was not statistically different from the baseline value (Table 3). The efficacy of TCZ was not significantly different according to the background DMARD therapy (data not shown).
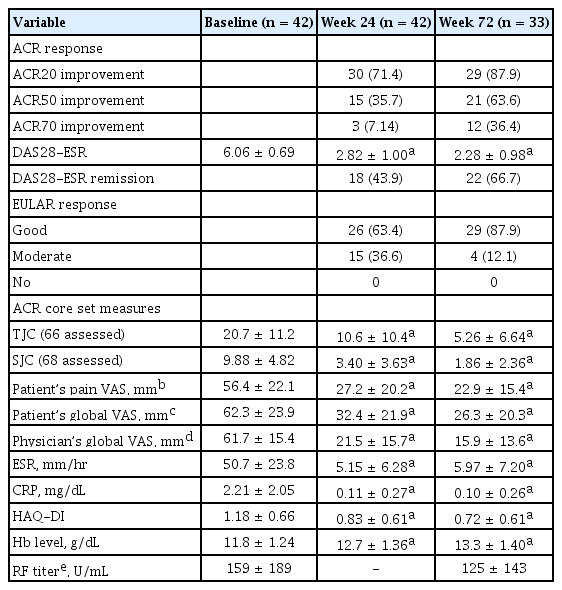
Clinical efficacy from those who were treated with tocilizumab successively during the main and extension study periods
Safety assessment
Main study
Ninety-nine patients who received TCZ (n = 48) or placebo (n = 51) at least once during the study were assessed for safety. The patients who experienced any AEs were more frequent in the TCZ group compared to the placebo group (89.6% vs. 60.8 %, p = 0.001). The occurrence of AEs associated with infection was 41.7% of the patients in the TCZ group, which was higher than those in the placebo group (23.5%, p = 0.0538) (Table 4).
TCZ treatment showed some changes in laboratory measurements at week 24. The patients in the TCZ group presented a decline in counts of leukocytes, neutrophils, and platelets at week 24 from baseline. However, the number of patients who decreased in cell counts from normal baseline to low values at week 24 differed significantly from the placebo group only in terms of leukocytes (17.5% vs. 0%, p = 0.016). The mean levels of aspartate transaminase (AST) and alanine transaminase (ALT) increased at week 24 in the TCZ group, but were within the normal range. An increase in the AST and ALT levels from the normal baseline to > 40 IU/L in the TCZ group was comparable to the placebo group. The concentrations of serum total cholesterol, low density lipoprotein (LDL), and high density lipoprotein (HDL) of the TCZ-treated patients were elevated at week 24 compared with those of placebos, although that of triglyceride (TG) was not. The numbers of patients who had an increase in the levels of lipid from normal baseline values were not statistically different between the TCZ and the placebo groups (Fig. 3).
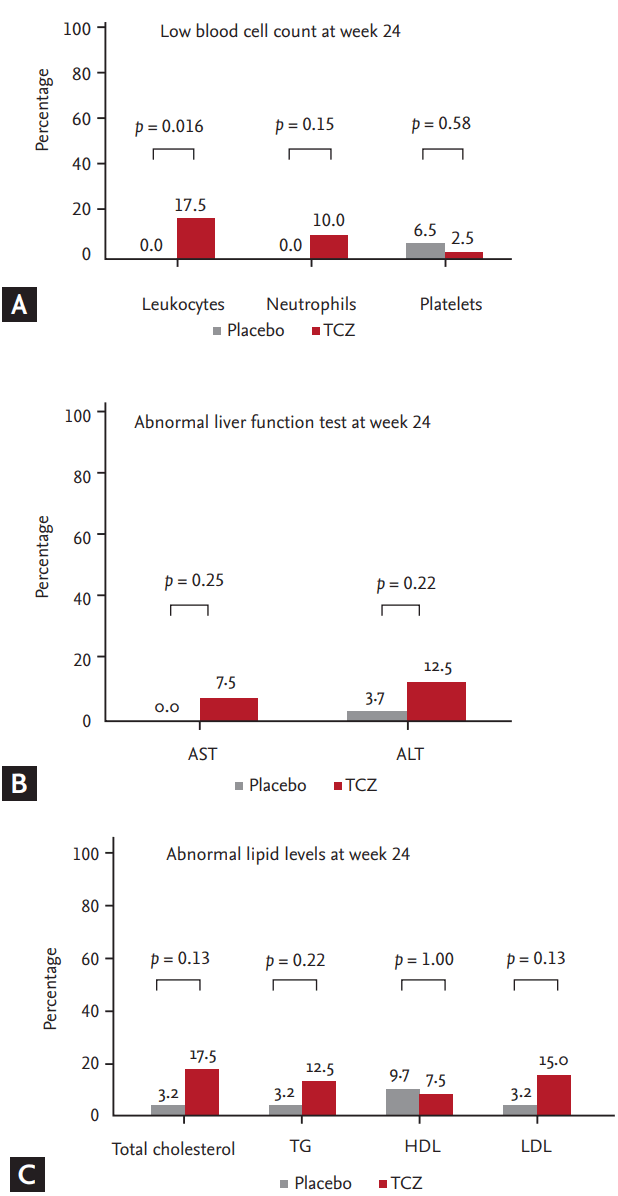
Laboratory measurements relevant to AE at week 24. (A) Percentage of patients who showed a decrease from normal baseline to low cell counts at week 24. (B) Percentage of patients who showed an increase from normal baseline to abnormal aspartate transaminase (AST) and alanine transaminase (ALT) levels at week 24. (C) Percentage of patients who showed an increase in lipid levels (total cholesterol ≥ 240 mg/dL; low density lipoprotein [LDL] ≥ 160 mg/dL; high density lipoprotein [HDL] ≥ 60 mg/dL; and triglyceride [TG] ≥ 500 mg/dL) at week 24 from normal baseline from the main study. TCZ, tocilizumab.
Extension study
Safety was assessed for 89 patients who were infused with TCZ once or more during the main or extension study period (Table 5). A total of 1,181 TCZ infusions were administered for 1.04 ± 0.31 years on average. Eighty-four patients (94.4%) experienced one or more AEs and the incidence of AEs was 5.28 per patient. Any ADRs occurred in 66 patients (74.2%) with the incidence of 3.35 per patient. Rate of AEs per 100 patients-years was 505.7 events and was highest during the first 3 months. Twenty-one AEs from 13 patients (14.6%) led to discontinuation of the study. The intensity of most AEs was mild (85.3%) or moderate (11.1%). Twenty-three patients (25.8%) had 29 serious AEs, of which 19 events from 14 patients (15.7%) were drug-related. These serious ADRs included nine infection-related events and others including gastric ulcer, increased AST, increased ALT, fracture, avascular bone necrosis, uterine cervical dysplasia, lung adenocarcinoma, thrombophlebitis, pulmonary embolism, and abortion. A 68-year-old woman with a ureteral stone died from sepsis due to urinary tract infection following 12 infusions of TCZ. Most frequent ADRs were pharyngitis (n = 21), increased ALT (n = 19), increased AST (n = 16), leukopenia (n = 17), granulocytopenia (n = 16), and hypercholesterolemia (n = 12).

Adverse events and adverse drug reactions in the patients who were treated with tocilizumab during the main or extension study period (safety population)
Forty-seven patients (52.8%) experienced 72 infection- related AEs (Table 5). Fifty-five events from 37 patients (41.6%) were drug-related. These included upper respiratory tract infection (n = 27) including pharyngitis, lung infection (n = 4) including bronchitis, pneumonia, and empyema, fungal dermatitis (n = 5), herpes zoster (n = 4), cellulitis (n = 3), cystitis (n = 3), herpes simplex (n = 2), and urinary tract infection (n = 2). Ten infusion reactions occurred in six patients (6.7%). These reactions developed within 24 hours following the end of drug administration. No one withdrew from the study due to these AEs.
Neutropenia, elevated AST or ALT level, and abnormal lipid profile were remarkable changes in laboratory measurements relevant to AE during the whole study period. Neutropenia at any time point was observed in 34 patients (38.2%), the majority of whom (n = 27) were of grade 1 or 2. The elevated level of AST above normal was observed during the study in 34 patients (38.2%). These elevations were < 3-fold the upper limit of normal, except for one patient. The level of serum ALT was elevated above normal upper limit in 45.4% (n = 39) of the patients with the initial normal level. Most had serum ALT level < 3-fold the upper limit of normal. The number of patients who had initially normal, but elevated levels of cholesterol (≥ 240 mg/dL), LDL (≥ 160 mg/dL), HDL (≥ 60 mg/dL), and TG (≥ 500 mg/dL) at week 72 was 6 (7.1%), 5 (5.6%), 22 (43.1%), and 0 (0%), respectively. Lipid ratios including total cholesterol/HDL, LDL/HDL, and non-HDL/HDL increased at week 72. Increases of total cholesterol/ HDL, LDL/HDL, and non-HDL/HDL by more than 30% were found in 12.4% (n = 11), 21.3% (n = 19), and 22.5% (n = 20) patients, respectively.
Parameters associated with DAS28 remission at week 72
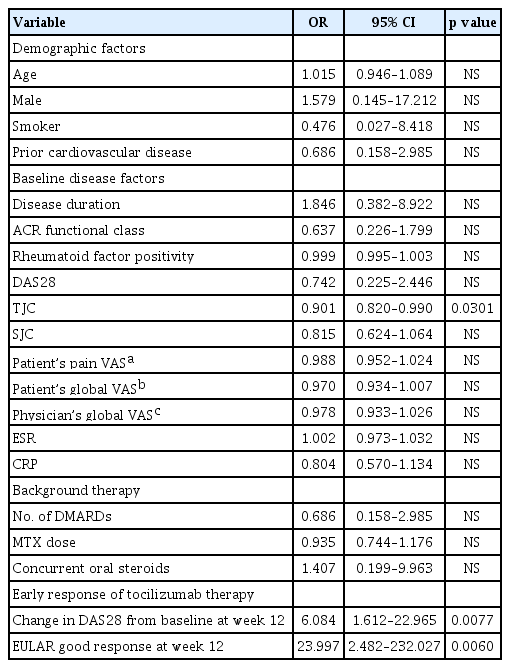
We looked for demographic or clinical parameters associated with DAS28 remission at week 72 by comparing the patients with remission and those without remission. Univariate analysis showed that the bigger change of DAS28 from the baseline at week 12, EULAR good response, and lower initial TJC were associated with DAS28 remission at week 72 (Table 6).
DISCUSSION
The results of the main 24-week trial demonstrated that add-on infusion of TCZ 8 mg/kg rapidly lead to clinical improvements in Korean patients with active RA refractory to conventional DMARD therapy including MTX. Significantly more patients with TCZ treatment achieved ACR20 and ACR50 responses, DAS28 remission, and EULAR good response with the improvements of symptoms, signs, and laboratory measurements, such as hemoglobin and acute phase reactants, compared with the control group at week 24. These results were generally comparable to previous reports from Japanese or Caucasians [7,9-11,20]. Most previous studies in which ACR70 achievement rate of TCZ treated patients was significantly superior to that of the placebo group. However, ACR70 response rate at week 24 was relatively low (4.3%) and did not differ from the placebo in the present study. The placebo group also showed lower ACR20/50/70 response rates compared with previous studies. ACR70 response rate similar to the results of previous studies was delayed until week 72 in our study. During the following 52-week extension study, the improvements at 24 weeks were maintained in the majority of the subjects. Clinical efficacy of added TCZ did not differ according to background DMARD therapy during the main and extension studies. A previous study reported no difference in ACR20 response according to the number of DMARDs as a background therapy [7].
The proportion of patients with ACR20/50/70 response, DAS28 remission, and EULAR good response increased continuously when they were maintained on TCZ treatment. Clinical remission, represented by DAS28 remission, was achieved by 67% of patients treated with TCZ infusion for 72 weeks. This population would benefit most from long-term TCZ treatment. DAS28 remission at week 24 has been associated with a younger age (< 55 years) [21]. Another study reported that short disease duration (< 4.8 years) and lower disease activity (DAS28 < 5.23) were predictors of remission at week 52 [22]. Presently, the change of DAS28 at week 12, EULAR good response, and initial TJC were associated with DAS28 remission at week 72 (Table 6). Thus, EULAR good response at week 12 can be a favorable predictor of DAS28 remission at week 72.
General safety profile in our study was drawn from 89 patients treated with TCZ once or more during the main or extension study period (Table 5). They had a total of 470 AEs, 29 serious AEs, and 21 AEs leading to discontinuation during a mean duration of 1.04 ± 0.31 years. These figures, roughly estimated, were higher compared with from a prior study in which overall rates of any AEs, serious AEs, and AEs leading to discontinuation in TCZ clinical trials 278.2, 14.4, and 5.8 events per 100 patient-years, respectively [23].
In terms of intensity of AE, our study more extensively collected AEs of mild to moderate intensity, rather than severe AE, as the frequency of severe AE was little different between our and previous reports.
As expected with the involvement of IL-6 in the immune response against infection [24] and previous reports on TCZ treatment [7,9-11,20,25], infection occurred more frequently in those receiving TCZ than controls during the study. Upper respiratory tract infection was the most common, and serious infection also occurred more often in the TCZ group. TCZ can mask signs of infection, such as fever and general malaise [26]. Thus, infection should be always monitored in patients treated with TCZ. Interferon γ (IFN-γ) plays a role in the immune response to tuberculosis. IL-6 production induced in response to infection by Mycobacterium tuberculosis inhibits the responsiveness of macrophages towards IFN-γ [27]. In addition, in vitro IFN-γ production in response to mycobacterial antigen exposure is not impaired by TCZ [28]. These observations suggest a low risk for tuberculosis reactivation during TCZ therapy. In this trial, pre-screening test for latent tuberculosis was performed; patients with latent tuberculosis were enrolled after appropriate treatment with anti- tuberculosis medication. There was no specific infection related with tuberculosis during the study period.
Two cases of malignancy or pre-malignant lesion occurred in the TCZ group. One was a lung adenocarcinoma and the other was uterine cervical dysplasia. IL-6 appears to be a potentially causative factor of malignant diseases [29]. The carcinogenesis risk of IL-6 cannot be excluded until further epidemiologic surveillance data are available.
The reduction in neutrophil count in patients receiving TCZ is consistent with that reported previously [9-11]. Neutropenia was usually mild and transient, and was not associated with infection or fever. Some possible mechanisms of lowered neutrophil count by TCZ include blocking IL-6-induced neutrophil survival, down-regulation of other inflammatory cytokines, and the migration of neutrophils from the circulation into tissues [30,31].
Most common AEs involved in liver and biliary system were hepatic enzyme elevations without any clinical symptoms in our study, consistent with previous reports [9-11]. These increases were generally mild and transient, with normalization occurred spontaneously or after temporary interruption of TCZ infusion.
Cardiovascular diseases are a major cause of mortality in patients with RA [32] and risk factors including lipid profile and their modification are a serious concern [33]. Although cardiovascular complications were rarely found in our study compared with previous studies [8,10], plasma lipid levels rose in the TCZ group. These increases might be associated with the control of disease activity. Patients with active RA often have lower lipid concentrations than the general population in association with the inflammatory process, and increases have been seen with improvement in chronic inflammation [34]. However, atherogenic indices, such as the LDL/HDL and non-HDL/HDL ratios, were elevated by > 30% from baseline values in about 20% of patients. Thus, long-term detrimental effect to cardiovascular system cannot be excluded in those receiving TCZ. The Japanese guideline recommends monitoring serum lipid level during TCZ treatment [35].
Decline in RF titer has been observed in patients with RA treated with TNF blocking agents [36,37] and it has been reportedly associated with treatment response [38]. However, there have been few reports on the alteration of serum RF level by the treatment with TCZ. From our study, serum RF titer was markedly decreased in the TCZ group compared to the placebo group at 24 weeks. This phenomenon was described in an earlier study on TCZ [11]. However, there was a contradictory report of no significant RF level change and no correlation between clinical response and RF levels [39]. The mechanism of the reduction in RF titer in response to biological treatment is not fully understood. IL-6 blockade might inhibit B cell autoantibody production as in TNF-blocking [40]. Interestingly, our extension study showed that serum RF titer at week 72 did not differ from the baseline. The initial decline of RF production by TCZ was not sustainable, but independent of long-term clinical response. There are no conclusive evidences so far on the reduction of RF level and/or its association with clinical improvement during TCZ therapy. It requires further investigation.
The current study does have limitations. The longterm effect of TCZ on structural damage and quality of life in patients with RA was not evaluated and it requires further studies. The efficacy of TCZ monotherapy was reported to be comparable to the combination of TCZ and DMARDs [41], but it remains to be confirmed in Korean patients in the future study. Many biologic agents including TNF blocking agents have been already used for patients with active RA refractory to conventional DMARD therapy in Korea. Comparative studies among biologic agents including TCZ will ascertain which one is better for the specified patients with active RA. Future studies should include the assessment of longer-term AEs which can occur 72 weeks after TCZ administration. It is also required to investigate how long TCZ efficacy is maintained and how to taper TCZ after the remission.
KEY MESSAGE
1. Tocilizumab (TCZ) infusion (8 mg/kg) add-on is highly efficacious in Korean patients with active rheumatoid arthritis (RA) refractory to conventional disease modifying anti-rheumatic drugs (DMARDs) including methotrexate (MTX).
2. DAS28 (disease activity score in 28 joints) remission was achieved in one-thirds of the patients at week 72 and associated with European League Against Rheumatism (EULAR) good response at week 12. Week 12 EULAR good response could be a predictor of the remission with long-term TCZ therapy.
3. TCZ with DMARDs including MTX is well-tolerated in the majority of patients during the 72 week treatment period, although TCZ therapy has possible adverse events including serious infection, abnormal liver function, atherogenic lipid profile, and malignancy.
Notes
This work was supported by JW Pharmaceutical Co. Ltd.