Seropositive rate of the anti-hepatitis A immunoglobulin G antibody in maintenance hemodialysis subjects from two hospitals in Korea
Article information
Abstract
Background/Aims
Hepatitis A virus (HAV) is a self-limiting infectious disease, but 1% of subjects develop fulminant hepatitis. The prevalence of the anti-HAV immunoglobulin G (IgG) antibody in hemodialysis subjects in Korea remains unknown. The purpose of this study was to describe and compare the seropositive rate of anti-HAV antibody among hemodialysis subjects in two hospitals according to age group.
Methods
A total of 170 hemodialysis subjects were evaluated for the seropositive rate of the anti-HAV IgG antibody and its titer.
Results
Of the 170 maintenance hemodialysis subjects in two hospitals (Kangnam 92 vs. Chuncheon 78), 79 (46.5%) were male. The mean age was 53.2 years old, and 94.1% of the subjects were over 40 years old. The median vintage of hemodialysis was 29.0 months. Anti-HAV antibody was found in 163 subjects (95.9%), with no significant difference between the two areas (Kangnam 97.8% [n = 90] vs. Chuncheon 93.6% [n = 73]). Subjects younger than 40 years old showed a seropositive rate of 50%, while the seropositive rate increased with age for subjects aged 40 or older (p for trend < 0.001). Seropositive subjects from Kangnam showed a higher anti-HAV antibody titer than those from Chuncheon (median: Kangnam 14.2 vs. Chuncheon 11.7). Only age influenced seropositivity. The only factor that influenced the antibody level was the location of hospital (p < 0.001).
Conclusions
The seropositive rate of the anti-HAV antibody in hemodialysis subjects was 95%, which is similar to findings in the general population. Active immunization against hepatitis A is strongly recommended for hemodialysis subjects under 40 years of age after anti-HAV testing.
INTRODUCTION
The hepatitis A virus (HAV) is a RNA virus transmitted through contaminated food and water, and tens of millions of individuals worldwide are estimated to be infected with HAV each year [1]. The clinical manifestation of hepatitis A is mostly gastrointestinal, including flu-like symptoms with mild jaundice, but it may progress to liver failure. It is known that the severity and the clinical course of HAV are related to the age at infection. Most children with HAV infection are asymptomatic, whereas in older children and adults the symptoms are more severe. If one develops acute hepatitis, the anti-HAV antibody provides immunity for the rest of one’s life.
The incidence rate of hepatitis A is associated with socioeconomic status and region [2,3]. In the past, most of the population would have the anti-HAV immunoglobulin G (IgG) antibody; however, through improvements in socioeconomic factors and public health, the seroprevalence of the anti-HAV IgG antibody has decreased, and more individuals are at risk of developing symptomatic hepatitis A [4]. In Korea, the seroprevalence of the anti-HAV IgG antibody has changed with improved socioeconomic conditions, and the incidence of overt acute hepatitis A in adults has recently increased [5-7].
Because hemodialysis subjects are immunocompromised and have a poor nutrition status, they are at a high risk for severe hepatitis [8]. Therefore, vaccination is necessary in hemodialysis patients without the anti-HAV antibody.
Most studies of the prevalence of hepatitis A have investigated the general population, and studies about hemodialysis are scarce. Although a few studies about hemodialysis have been conducted, they all focused on the effect of vaccines or the incidence of hepatitis A, and no report has described the prevalence of anti-HAV antibody in hemodialysis subjects [9,10]. Likewise, no study has analyzed regional differences in hemodialysis subjects.
In present study, the authors investigated the prevalence of the anti-HAV IgG antibody in hemodialysis subjects and compared these findings between subjects from two different hospitals in Korea.
METHODS
Study population
A total of 170 hemodialysis subjects in Hallym University Kangnam Sacred Heart Hospital in Seoul (Kangnam) and Hallym University Chuncheon Sacred Heart Hospital in Gangwon province (Chuncheon) were enrolled, according to the following inclusion criteria: (1) subjects who had maintained hemodialysis for more than 3 months; (2) were more than 20 years old; and (3) understood the purpose of this study and provided written consent. Our exclusion criteria were as follows: (1) subjects who had a fever (above 38°C) and an acute infection within 2 weeks; (2) subjects who were pregnant or had the potential to become pregnant; and (3) subjects who had acute hepatitis within 3 months. Demographic data such as age, sex, the cause of hemodialysis, and vintage of hemodialysis were collected and evaluated in comparison with the general population.
The protocol was approved by the Institutional Review Boards of all participating centers (IRB No. 2010-12-82) and all investigations were performed according to the guidelines of the Helsinki Declaration. Written informed consent was obtained from all participants.
Anti-HAV IgG antibody and titer measurements
We sampled 3 cc of blood before hemodialysis through arteriovenous access. The blood sampling was performed over the course of 2 weeks in each hospital and the anti-HAV IgG antibody was tested using a chemiluminescent microparticle immunoassay machine at the Green Cross Corporation (Youngin, Korea). The cut-off for seropositivity was over 1.0 (S/CO: signal to cutoff: ratio of the sample reactive light unit [RLU] to the cutoff RLU).
Statistical analysis
The seropositive rate of the anti-HAV IgG antibody was analyzed as a categorical variable, as positive (with antibody) or negative (without antibody). The total seropositive rate was analyzed in terms of frequency. Continuous variables, such as age, vintage of hemodialysis, and anti-HAV antibody titer, were analyzed by the Student t test, Kruskal-Wallis test, Mann-Whitney U test, or Jonckheere-Terpstra test as appropriate. Categorical variables such as sex, cause of end-stage renal disease (ESRD), and age group were analyzed by the chi-square test. A multivariate logistic regression model was created to analyze the risk factors for seropositivity among hemodialysis subjects. A 2-sided p ≤ 0.05 was considered to indicate statistical significance. Statistical analysis was conducted using SPSS version 22 (IBM Co., Armonk, NY, USA).
RESULTS
Baseline characteristics of the subjects according to area
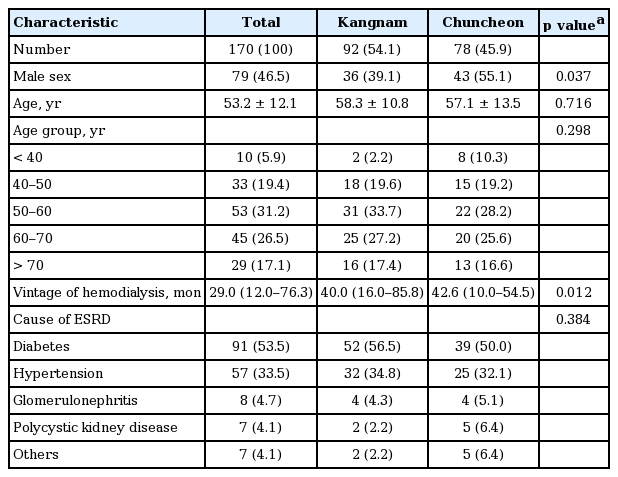
A total of 170 maintenance hemodialysis subjects from the two hospitals were enrolled in this study. Among them, 79 (46.5%) were male. The mean age was 53.2 years old, and 94.1% of the subjects were over 40 years old. The median vintage of hemodialysis was 29.0 months. The causes of ESRD were as follows: diabetes, 91 (53.5%); hypertension, 57 (33.5%); glomerulonephritis, eight (4.7%); and polycystic kidney disease, seven (4.1%). Men were more prevalent in Chuncheon (Kangnam vs. Chuncheon, 36 [39.1%] vs. 43 [55.1%]), and the vintage of hemodialysis was greater among the subjects from Chuncheon (Kangnam vs. Chuncheon, 40 [interquartile range (IQR), 16.0 to 85.8] vs. 42.6 [IQR, 10.0 to 54.5]). Significant differences in age, distribution of age groups, and cause of ESRD were not observed between the two groups (Table 1).
Seropositive rate of the subjects according to hospital
Of the 170 subjects, 163 (95.9%) had the anti-HAV IgG antibody. There was no difference in the presence of the antibody between men and women (men vs. women, 75 [94.9%] vs. 88 [96.7%], p = 0.563) and the antibody titer was likewise not significantly different (men vs. women, 12.3 [IQR, 10.7 to 14.3] vs. 13.1 [IQR, 11.7 to 14.7], p = 0.127).
In Kangnam hospital, 90 subjects (97.8%) were seropositive, compared to 73 (93.6%) in Chuncheon hospital. The seropositive rate was not significantly different between the two hospitals (p = 0.166) (Fig. 1). Among all subjects, regardless of hospital, subjects younger than 40 years old showed a seropositive rate of 50%, while subjects aged between 40 and 50, between 50 and 60, and 60 or older showed seropositive rates of 97%, 98%, and 100%, respectively. As age increased, the seropositive rate increased (p for trend < 0.001). However, there was no difference in the prevalence by age group between the two hospitals.

Seropositive rate of anti-hepatitis A virus (HAV) immunoglobulin G antibody (A) by age group and (B) by age group and hospital.
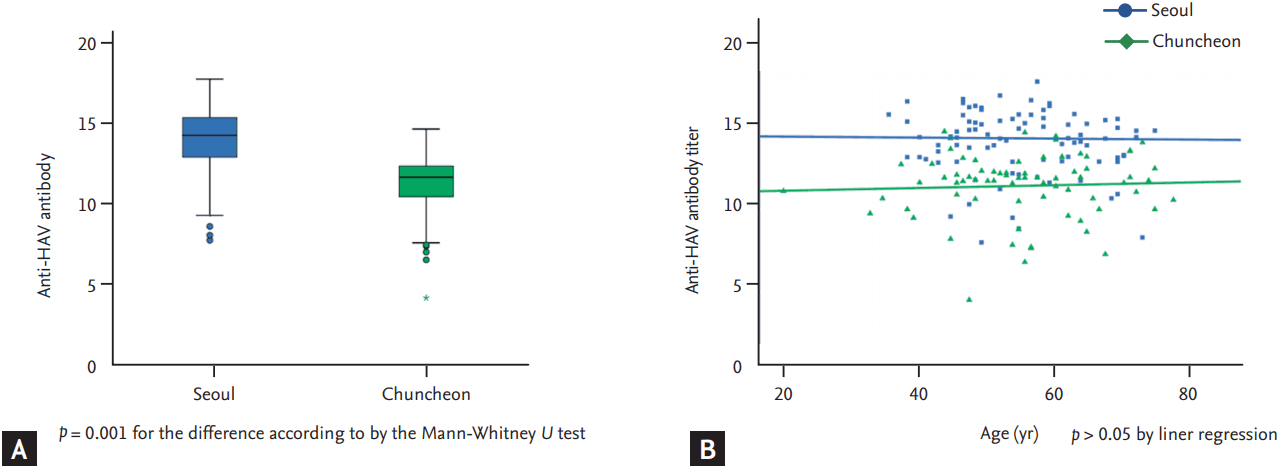
The median anti-HAV IgG antibody titer among HAV antibody-positive subjects was 13.0 (IQR, 11.6 to 14.6). Because the titer was not normally distributed, the analysis was done using non-parametric statistics. The level of anti-HAV IgG antibody was higher in Kangnam (Kangnam vs. Chuncheon, 14.2 [IQR, 12.9 to 15.3] vs. 11.7 [IQR, 10.0 to 12.4], p < 0.001) (Fig. 2). However, a change in titer by age in the linear regression model was not observed (p > 0.05 in the Kangnam and Chuncheon).
Relationship between the vintage of hemodialysis and seropositive rate or titer of the subjects
The factors associated with seropositivity of the anti-HAV antibody were examined using a multiple logistic regression model. Age, hypertension as a cause of ESRD, and vintage of hemodialysis were found to be significant factors in the univariate analysis. An analysis was then performed using a multiple logistic regression model by adding sex. In this model, hypertension, vintage of hemodialysis, and sex were not found to be significant risk factors; instead, only age was confirmed to be a significant factor related to anti-HAV seropositivity (Table 2).
DISCUSSION
In summary, most maintenance hemodialysis subjects aged 40 or older were positive for the anti-HAV IgG antibody. There was no difference between the two hospitals in seropositivity for the anti-HAV antibody. Mean-while, only approximately 50% of hemodialysis subjects aged less than 40 were positive for the anti-HAV IgG antibody. The anti-HAV IgG antibody positivity rate increased with age. However, no association was observed with the duration of hemodialysis or cause of ESRD, other than age. Interestingly, the anti-HAV titer was found to be significantly higher in Kangnam hospital in Seoul. In the present study, subjects under 40 had a lower seropositive rate, while subjects older than 40 years showed a nearly 100% rate. Several studies of the general population have shown that younger subjects, in their 20s and 30s, had an anti-HAV IgG antibody seroprevalence of approximately 10% to 35%, whereas the corresponding group of maintenance hemodialysis subjects in this study showed a seropositive rate of approximately 50% [11,12]. Elderly subjects in this study had a similar seropositive rate to that of the general population. Therefore, it is desirable to encourage HAV vaccination in hemodialysis subjects younger than 40 according to the latest guidelines.
Gangwon-do in Korea, since 1995, the incidence of acute symptomatic hepatitis in adults has rapidly increased, and in the 2000s, hepatitis A comprised 70% to 80% of cases of symptomatic acute hepatitis [13-15]. Several studies have reported the most common age range for HAV infection to be 10 to 29 years [16-18]. Thus, the young population is exposed to a high risk of HAV infection.
In hemodialysis subjects, the risk of HAV infection is greater because they are immunocompromised, putting them at risk of severe fulminant hepatitis [10,19,20]. Additionally, the frequency of travel among hemodialysis subjects has increased; thereby, increasing the chances of HAV infection, although this infection is not transmitted parenterally. Therefore, in connection with the current decreased seropositive rate of young subjects and the increasing incidence of active hepatitis, it is advisable to vaccinate hemodialysis subjects against HAV. A study in 2008 reported that subjects with HAV infection in the 20 to 39 age group accounted for 71.3% of total cases of infections and 74.6% of medical costs [21]. Thus, active immunization of under 40-year-old hemodialysis subjects could be beneficial from the perspective of medical costs. The response to vaccines in hemodialysis subjects is generally suboptimal due to their immunocompromised status, which is associated with dialysis, underlying disease, and medication [22,23]. However, vaccination against HAV in hemodialysis subjects shows a good response, similar to that of the normal population, and is far better than the response to hepatitis B vaccination [19].
This study showed that gender, hospital, and the cause of ESRD were not associated with the seropositive rate of the anti-HAV IgG antibody. Previous studies of the general population have reported similar results [12,24]. HAV infections are closely related with geographical endemicity, and even in the same country, it presents differently across regions. However, no difference in the seropositive rate was found between the two hospitals in the different areas, Seoul and Gangwon-do. A nationwide study would be needed for the evaluation of geographic differences in the seropositive rate of the anti-HAV IgG antibody.
Although there was no difference in seropositive rate between the two hospitals, the antibody level did differ. Age was the only factor found to influence the antibody titer in the additional analysis. Even though a very low titer of antibody is protective against HAV infection, it is interesting that the titer varied by hospital. Further analysis of the factors that influence antibody titer is necessary in the future.
Our study has limitations. This study was performed among subjects in two general hospitals in different areas; however, the two hospitals may not have been representative of each region and we did not take into account their socioeconomic status. Among the total study population (n = 170), the amount of subjects under 40 years old was very small (n = 10), which makes it difficult to make a comparison between the age groups. Finally, there was no investigation into whether subjects had been vaccinated against HAV.
In conclusion, this study described the seropositive rate of HAV in Korean hemodialysis subjects for the first time. The seropositive rate of the anti-HAV IgG in maintenance hemodialysis subjects in Korea was over 95%, which was not significantly different from that of the general population. Testing for the anti-HAV IgG antibody and consecutive active immunization is strongly recommended for ESRD subjects under 40 years of age.
KEY MESSAGE
1. Most maintenance hemodialysis subjects aged 40 or older were positive for the anti-hepatitis A virus (HAV) immunoglobulin G (IgG) antibody, with no significant difference between the Kangnam and Chuncheon.
2. Fifty percentage of hemodialysis subjects younger than 40 were positive for the anti-HAV IgG antibody.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This research was supported by a grant from the National Research Foundation, Republic of Korea (grant number: NRF-2016R1D1A1B03934173).