Palliative and end-of-life care for heart failure patients in an aging society
Article information
Abstract
The populations of Asian countries are expected to age rapidly in the near future, with a dramatic increase in the number of heart failure (HF) patients also anticipated. The need for palliative and end-of-life care for elderly patients with advanced HF is currently recognized in aging societies. However, palliative care and active treatment for HF are not mutually exclusive, and palliative care should be provided to reduce suffering occurring at any stage of symptomatic HF after the point of diagnosis. HF patients are at high risk of sudden cardiac death from the early stages of the disease onwards. The decision of whether to perform cardiopulmonary resuscitation in the event of an emergency is challenging, especially in elderly HF patients, because of the difficulty in accurately predicting the prognosis of the condition. Furthermore, advanced HF patients are often fitted with a device, and device deactivation at the end of life is a complicated process. Treatment strategies should thus be discussed by multi-disciplinary teams, including palliative experts, and should consider patient directives to address the problems discussed above. Open communication with the HF patient regarding the expected prognosis, course, and treatment options will serve to support the patient and aid in future planning.
INTRODUCTION
Population aging in Asia
The development of advanced medical care techniques and life-saving procedures has greatly extended life expectancy. Currently, 8.5% of the world’s population is over 60 years old, accounting for 617 million people, and this number is expected to increase more than threefold, to 2 billion, by 2050, while the global life expectancy is estimated to extend by 8.4 years, from 68.6 to 76.2 years [1,2].
Notably, more than half of the world’s aged population lives in Asia [2,3]. Japan has the oldest population, with 26.6% of its citizens aged over 65 years in 2016, compared with only 13.1% in Korea [4,5]. However, the rate of population aging (i.e., the percentage of the population aged over 65 years) is expected to increase from 7% to 14% within 18 years in Korea, 20 years in Singapore, and 23 years in China [6]. Population aging is thus expected to advance more rapidly in other Asian countries compared with Japan, with the proportion of people aged over 60 in 2050 projected to reach 42.4% in Japan (1st in the world), 41.6% in Korea (4th), and 40.1% in Singapore (9th) (Fig. 1) [2].
Heart failure pandemic in Asia
The incidence of heart failure (HF) increases with age [7-9]. The number of HF patients has thus increased sharply, to an estimated total 26 million people worldwide, in line with the rapid increase in population aging [10,11], including in Asia [3,12,13]. The number of HF outpatients in Japan was estimated at 979,000 in 2005, and is predicted to increase gradually and reach 1.3 million by 2030 [14]. Similarly, the prevalence of HF in Korea was 0.75% in 2002, and is expected to approximately double (1.53%) by 2013 and then again (3.35%) by 2040 [15,16].
HF is a clinical syndrome caused by structural and/or functional cardiac abnormalities, with shortness of breath on exertion or peripheral edema being the main symptom, often accompanied by a significant reduction in quality of life (QOL) [17,18]. Because HF stage advances with aging, deterioration of QOL becomes more marked and the intensity of care required thus increases [19-21]. The explosive increase in the number of HF patients in Asia in association with pronounced population aging is likely to impose a heavy burden, not only economically but also socially, including with respect to palliative and end-of-life care for patients with advanced HF [21-27].
PALLIATIVE AND END-OF-LIFE CARE
Definitions of palliative and end-of-life care
The World Health Organization defined palliative care as ‘‘an approach that improves the QOL of patients and their families facing the problem associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial and spiritual’’ [28]. Palliative care can thus be considered an intensive and comprehensive approach to the patient’s physical, emotional, psychological, and mental care, providing support both to the patient and their caregivers [29].
End-of-life care refers to the support and medical care provided during the time near death [30]. However, the concept of end of life is complicated and definitions differ among reports [31]. A statement on end-stage cardiovascular care by the Japanese Circulation Society defined ‘end-stage’ as refractory HF conditions despite maximal medical therapy (i.e., stage D) and ‘end of life’ as the phase of life in which a person is living with an illness that will worsen and eventually cause death [30,32-34]. Lamont introduced the concept of life expectancy and considered the end-of-life period according to two perspectives: (1) a disease-centered perspective, in which the end-of-life period is one of irreversible decline before death, and (2) a time-based perspective related to the hospice admission criteria of a life expectancy of 6 months or less [35].
When should palliative and end-of-life care be started in HF patients?
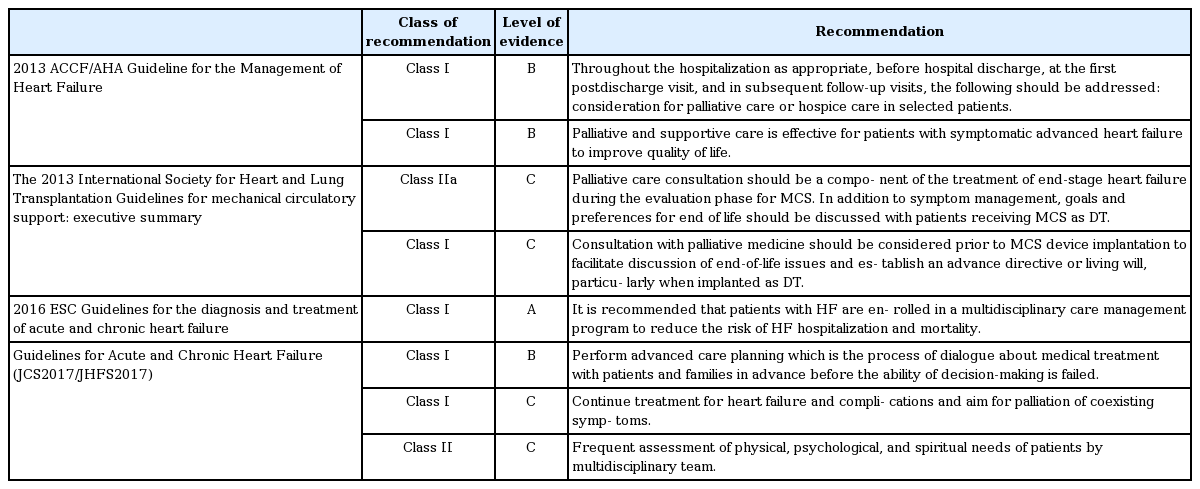
In recent years, numerous guidelines for palliative and end-of-life care have been recommended, based on accumulated evidence (Table 1). The American College of Cardiology Foundation/American Heart Association HF guidelines recommend palliative and supportive care to improve QOL for advanced HF patients, and as a treatment goal for patients with refractory conditions classified as stage D [33,36,37]. Another study recommended use of a quality index for the early detection of candidate HF patients for palliative care [38]. Candidates should fulfil seven criteria of the index, including severe symptoms (classified as New York Heart Association [NYHA] functional class IV), frequent hospitalization (> 3 times a year), and a general deterioration in clinical condition (e.g., presence of edema, orthopnea, nycturia, or dyspnea). A previous study concluded that HF patients should be considered as candidates for palliative care if a physician answered “no” to the question of “Would I be surprised if my patient were to die in the next 12 months?” [39]. Many treatments for HF can be considered as care leading to symptomatic relief. For instance, diuretic treatment for pulmonary congestion improves dyspnea in HF patients and thus alleviates the fear of death. Palliative care can therefore be provided at any stage of symptomatic HF after diagnosis to minimize suffering, in terms of physical pain and mental, social, and spiritual distress [20,22,40].
The European Society of Cardiology HF guidelines recommend that end-of-life care should be considered for patients meeting the following criteria: (1) progressive functional decline (physical and mental) and dependence in most activities of daily living; (2) severe HF symptoms with poor QOL despite optimal pharmacological and non-pharmacological therapies; (3) frequent admissions to hospital or other serious episodes of decompensation despite optimal treatment; (4) heart transplantation and mechanical circulatory support ruled out; (5) cardiac cachexia; and (6) clinically judged to be close to the end of life [17].
As noted above, palliative and end-of-life care are not identical concepts. Unfortunately, a structured qualitative interview of medical professionals, including doctors, revealed that care providers had limited comprehension regarding how palliative care could complement medical therapies and the value of palliative care for HF patients and care teams [41]. In addition, many medical professionals mistakenly believed that palliative care should only be provided to end-of-life patients facing death. End-of-life care is actually a specific type of palliative care provided for patients with an expected lifespan of < 6 months, designed to maintain daily QOL, avoid repeated hospitalization, and achieve a happy end of life, according to each patient’s wishes [40]. However, it is difficult to predict the mortality of HF patients accurately [42,43], and this prognostic uncertainty makes it difficult to plan towards fulfilling the patient’s end-of-life wishes. Palliative care should aim to meet the patient’s needs regardless of their prognosis (Fig. 2) [44,45].
MANAGEMENT OF PALLIATIVE CARE FOR HF
The World Health Organization estimates that 39% of the 40 million people with cardiovascular disease worldwide require palliative care on a yearly basis [46], but unfortunately 86% of them do not receive this care. It is necessary to properly address the various symptoms that manifest in HF (Table 2).
Dyspnea
Dyspnea is one of the main features of congestive HF. The initial management of dyspnea in HF patients includes standard treatment with diuretics, vasodilators, and positive inotropic drugs as necessary. Thoracic puncture may be performed for refractory pleural effusion, while oral or intravenous morphine may alleviate symptoms in patients with refractory dyspnea. However, most patients dying of HF are less likely to be alleviated using opioids due to repulsion [47]. Adaptive servo-ventilation therapy was recently reported to have a negative effect on prognosis among HF patients with reduced ejection fraction [48], while oxygen therapy and appropriate positive pressure ventilation may be useful for symptomatic relief in some cases [49].
Pain
Pain has been reported in up to 89% of HF patients classified as NYHA functional class IV [50]. In addition, moderate to severe pain was noted in 41% of patients dying of HF during their final 3 days, with overall pain reported in approximately 80% of patients dying of HF [51]. Similar to the symptomatic relief of dyspnea, low-dose opioids may help control pain in this population. Nonsteroidal anti-inflammatory agents are not recommended because they tend to cause sodium retention, gastrointestinal bleeding, or deterioration of renal function [52].
Fatigue
General fatigue may result from decreased cardiac output, and positive inotropic agents may provide symptomatic relief. Continuous intravenous administration of inotropes has been associated with a reduced need for hospitalization in cases of worsening HF, but could increase mortality [53]. Furthermore, fatigue may be related to depression, which co-exists with HF in many patients. However, the efficacy of selective serotonin reuptake inhibitors for treating depression in HF patients has not been established [54].
Psychosocial and spiritual problems
Patients with advanced HF often suffer from psychosocial and spiritual problems, characterized by hopelessness, isolation, and altered self-image [55]. They may have anxiety over issues such as the meaning of life, their physical needs, their family and social life, loss of dignity, increased dependence, and a desire to die [56]. To address these problems, it is necessary to discuss subjects such as life goals and life closure, illness and attendant suffering, coping ability, etc., with both the patients and their caregivers [57].
END-OF-LIFE CARE FOR PATIENTS WITH DEVICE THERAPY
Implantable cardioverter-defibrillator
Patients with HF at high risk of sudden cardiac death (SCD) due to ventricular arrhythmia may be fitted with an implantable cardioverter-defibrillator (ICD) [58]. Recent guidelines recommend this option in patients with a life expectancy of > 1 year [17,59]. However, ICDs are more likely to cause serious distress and anxiety in patients with advanced HF due to frequent shocking [60,61], and ICD deactivation is therefore a recommended treatment option during end-of-life care in patients with stage D HF.
Although most clinicians understand the need to consider ICD deactivation, many are reluctant to discuss this issue with their patients; indeed, in one study, only 25% of patients’ families discussed switching off their ICD device with the physician before the patient died [62]. Unfortunately, even in patients who had previously expressed a wish to restrict life-prolonging therapy, ICD deactivation was only discussed near the end of life. Clinicians thus need to discuss device deactivation with end-stage HF patients at an earlier and more appropriate stage.
Ventricular assist devices
Patients with advanced HF who are not eligible for heart transplantation may be suitable for an implantable artificial heart (ventricular assist device [VAD]). The 48-month survival rate of patients with a VAD is 49% in the United States, while it is especially high (88.7%) in Japan [63,64]. However, VAD implantation involves invasive thoracotomy and hospitalization, which are associated with a high risk of complications such as stroke and infection [65,66], especially in older patients [67]. Furthermore, although a VAD can relieve HF symptoms, such as shortness of breath and dyspnea, and improve exercise capacity, patients may continue to experience other symptoms such as physical pain, depression, and anxiety [68].
VADs also involve extensive commitment from the patient’s caregivers, who are required to provide 24-hour support for an indefinite period after VAD transplantation. Further research is needed to establish an effective strategy to reduce this burden on caregivers [22].
VAD implantation should only be performed after extensive discussion among medical professionals, patients, and their caregivers. Ideally, the VAD care team should also include palliative specialists to assist with pre- and post-operative pain or symptom management, and support the transition to end-of-life care if necessary.
ISSUES WITH PALLIATIVE AND END-OF-LIFE CARE IN THE ELDERLY
Most hospitalized patients with worsening HF are elderly, with an average age of patients in the REALITY-AHF (Registry Focused on Very Early Presentation and Treatment in Emergency Department of Acute Heart Failure) registry in Japan of 78 years [69]. Palliative care for the elderly is generally considered to be difficult [70] for several reasons. First, elderly patients may have difficulty conveying their symptoms accurately and it may be hard for physicians to evaluate their pain. In addition, elderly patients are also more likely to have various comorbidities and to be receiving multiple drugs, and at high doses. Finally, the patient's individual circumstances have a major influence on the choice of treatment strategy, and it is necessary to involve caregivers in this discussion.
The end-of-life process in elderly patients is also more diverse and complicated than in younger adults [71], and views of life and death among the elderly vary. Contrary to expectations, most elderly patients in one study opted for longevity above QOL, and half wanted to be resuscitated as necessary [72]. However, such patients feel close to death for the remainder of their life and thus tend to explore the meaning of life. Most elderly patients with HF are prepared to confront their terminal condition, and although their wishes in terms of end-of-life care may change due to various factors, such as their degree of cognitive function and their surrounding environment, the desire for a tranquil death remains constant [73].
CARDIOPULMONARY RESUSCITATION
SCD can occur not only in patients with advanced HF, but also in those with early-stage HF [74]. SCD is the predominant cause of death in patients with less-symptomatic or less-severe HF, whereas progressive HF with hypotension, hypoperfusion, or metabolic disturbance is a more common cause of death among patients with advanced HF [75,76]. It is therefore important for all HF patients to consider, in advance, what treatment they would/would not like to receive if cardiopulmonary resuscitation is needed [77,78].
Physician Orders for Life-Sustaining Treatment (POLST) is a concept advocated in the United States based on practical experience of advance directives, and includes the do-not-attempt-resuscitation (DNAR) directive, which precludes cardiopulmonary resuscitation in the event of cardiac arrest [78,79]. Numerous POLST-like programs, such as Medical Orders for Life-Sustaining Treatment (MOLST), Medical Orders for Scope of Treatment (MOST), Transportable Physician Orders for Patient Preferences (TPOPP), Iowa Physician Order for Scope of Treatment (IPOST), Louisiana Physician Orders for Scope of Treatment (LaPOST), and Physician Order for Scope of Treatment (POST), have since been proposed and used in various parts of the United States. In contrast, the infrastructure required to ensure smooth operation of POLST has not been established in Japan, and the Ethics Committee of the Japanese Society of Intensive Care Medicine decided not to introduce POLST into acute care, concluding that correct understanding and application of DNAR should be given priority [80].
COMMUNICATION AND PLANNING FOR END OF LIFE
Advance care planning
As noted above, it is difficult to accurately predict the prognosis of HF. In addition, approximately 70% of patients lack decision-making capacity at the end of life [81]. It is therefore necessary for the expected prognosis to be reviewed repeatedly and discussed among medical professionals, patients, and their caregivers to ensure that the patient’s philosophy and goals in life are respected [82]. Advance care planning (ACP) is a voluntary process involving discussion about future care between an individual and their care providers, irrespective of discipline [83]. The goal of ACP is to help ensure that people receive medical care that is consistent with their values, goals, and preferences during serious and chronic illness [84]. The patient’s intentions with respect to ACP may be conveyed via a written advance directive in case of emergencies; however, in many patients with HF discussion of their ACP will be delayed until the terminal stage [85,86]. In addition, human emotions and intentions can fluctuate greatly, and medical professionals and caregivers are expected to guide the patient to make the best decisions at all stages of HF.
Palliative and end-of-life care team approach for HF
End-of-life care includes not only symptomatic care but also hospice care, advanced instructions, and ACP [32]. To address these complicated issues, it is discussion is necessary among a team that includes cardiovascular physicians, palliative medical doctors, psychiatrists, nurses, psychologists, patients, and their caregivers. In many cases, palliative care teams may have extensive experience in caring for cancer patients but limited experience with HF patients. In addition, cardiologists and HF specialists often do not feel comfortable and/or have not been trained to address the challenges associated with terminal care, while general physicians, geriatric physicians, and other clinicians with expertise in palliative care lack knowledge and expertise regarding HF management [32,87,88]. Furthermore, the patients themselves may not appreciate the life-limiting nature of HF and their prognosis presents an additional barrier to planning end-of-life care [89]. Maintaining open channels of communication about the anticipated prognosis, course, and treatment possibilities of HF is thus crucial to allow patients to plan for their future [90]. Furthermore, a team familiar with palliative care should be established early during the treatment of HF patients.
CONCLUSIONS
Given the aging population in Asia, palliative and endof-life care for elderly patients with HF is expected to become an even greater issue in the future. Withholding or discontinuing medical treatments during end-of-life care should be discussed by multidisciplinary teams, including palliative specialists, with consideration of the individual patient’s directives. Open communication with the HF patient regarding their expected prognosis, course, and treatment options will serve to comfort the patient and aid in planning future treatment.
Notes
No potential conflict of interest relevant to this article was reported.