Age at menarche and risk of colorectal adenoma
Article information
Abstract
Background/Aims
Limited data are available regarding the association between age at menarche and the risk of colorectal adenoma. Therefore, we aimed to evaluate the relationship between reproductive factors including age at menarche and the risk of colorectal adenoma.
Methods
A cross-sectional study was performed on asymptomatic female subjects who underwent colonoscopy between 2010 and 2014 as part of a comprehensive health screening program in Korea. The association between reproductive factors including age at menarche and the presence of adenomas was assessed using multivariate logistic regression analysis.
Results
Among 32,620 asymptomatic female subjects, the proportion of patients with menarche at 10 to 11, 12 to 13, 14 to 15, 16 to 17, and 18 to 19 years of age was 4.1%, 31.7%, 45.4%, 14.9%, and 4.0%, respectively. Age at menarche was not significantly associated with the risk of any adenoma (adjusted odds ratio [AOR], 0.99; 95% confidence interval [CI], 0.97 to 1.02; p = 0.500) or advanced adenoma (AOR, 0.98; 95% CI, 0.91 to 1.04; p = 0.468) after adjusting for confounding factors. Age at menarche was not significantly associated with the risk of adenoma even among similar age groups. In addition, parity, use of female hormones, and menopause were not associated with the risk of adenoma.
Conclusions
Age at menarche, parity, use of female hormones, and menopause were not significantly associated with the risk of colorectal adenoma. Our findings indicate that reproductive factors including age at menarche do not affect the development of colorectal adenoma.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer worldwide and its incidence is rapidly increasing in Asian countries [1-5]. Overall, the incidence and mortality rates of CRC are about 30% to 40% higher in men than in women and the median age at diagnosis is lower in men than in women (69 years old vs. 73 years old, respectively) [6]. Colorectal adenomas are the precursors of CRC, and also occur more commonly in men than in women [7,8]. The reasons for this discrepancy are not completely understood, but could be due to sex-specific differences in exposure to hormones [9,10]. Namely, female hormones may play a protective role in colorectal carcinogenesis, and indeed several researchers have proven this hypothesis [11,12].
Age at menarche has been used as an indicator of lifetime exposure to endogenous female hormones through cyclic ovarian function, and also marks the initiation of hormone changes from childhood to adolescence [13]. In addition, early menarche is associated with a more rapid onset of ovulatory cycles and a tendency to sustain higher levels of luteal phase estradiol [14]. Therefore, early menarche is thought to be associated with a greater lifetime exposure to female hormones, while later menarche is associated with less exposure to female hormones. Based on the potential ability of female hormones to reduce colorectal carcinogenesis described in previous experimental studies, we speculated that later age at menarche is associated with an increased risk of colorectal neoplasia (CRN).
To date, several studies have investigated the association between age at menarche and CRC [15-19]. Some studies have suggested inverse associations [15,16], whereas others have shown positive or no significant associations [17-19]. In other words, the epidemiological evidence for a causal relationship between age at menarche and CRC risk has been inconsistent. Moreover, little is known regarding the influence of reproductive factors including age at menarche on the risk of colorectal adenomas. Therefore, we aimed to evaluate the relationship between reproductive factors including age at menarche and the risk of colorectal adenomas in a very large sample of asymptomatic female patients.
METHODS
Study population
We retrospectively analyzed data obtained from a prospectively established cohort. The present study population consisted of asymptomatic female subjects aged ≥ 30 years old who had undergone a colonoscopy as part of a comprehensive health screening program at Kangbuk Samsung Hospital, Seoul and Suwon, Korea, between 2010 and 2014 (n = 53,029). The purpose of the screening program was to promote health through a regular checkup and to improve early detection of existing diseases. In Korea, the Industrial Safety and Health Law requires employees to participate in annual or biennial health examinations. Approximately 80% of the participants were employees of various companies and local governmental organizations and their spouses, with the remaining participants registering individually for the program. Before colonoscopy, interviews by general practitioners were conducted to ensure that all participants were asymptomatic (i.e., no lower abdominal pain or hematochezia). Participants with intestinal symptoms were urged to seek medical care.
For this analysis, the exclusion criteria were as follows: repeated data of subjects who underwent more than two colonoscopies (n = 2,964), a history of CRC or colorectal surgery (n = 136), a history of inflammatory bowel disease (n = 93), poor bowel preparation (n = 5,948), lack of an adequate biopsy (n = 79), missing data on age at menarche (n = 11,158), and diagnosis of CRC during the study (n = 31). A total of 32,620 eligible subjects were enrolled in the final analysis (Fig. 1).
This study was approved by the Institutional Review Board of Kangbuk Samsung Hospital (IRB number: 2016-04-002, approved on 2016-04-11). The requirement for informed consent was waived because only de-identified data were retrospectively accessed.
Measurements and definitions
Data on reproductive factors were collected through a standardized, self-administered questionnaire that asked about the regularity and frequency of menstrual periods, menopausal status, and use of female hormones including oral contraceptives and hormone replacement therapy. Age at menarche was defined as the age at the first menstrual period (in years). The question asked was “At what age did your menstrual periods begin (please state the age in full)?” and response categories were “10 or younger, 11, 12, 13, 14, 15, 16, 17, 18, 19, and 20 or older.” Parity was derived from the number of reported live births and stillbirths.
Data on medical history and health-related behaviors were also collected through a standardized, self-administered questionnaire, and physical measurements such as height and weight were performed by a trained staff member. Smoking status was categorized as never, formerly, or currently, and family history was defined as CRC in one or more first-degree relatives at any age. Self-reported use of nonsteroidal anti-inflammatory drugs (NSAIDs) (regular use over the previous month) was also assessed.
Hypertension was defined as a systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or current use of antihypertensive medication. Diabetes mellitus was defined as a fasting blood glucose ≥ 126 mg/dL, hemoglobin A1c ≥ 6.5%, or current use of insulin or antidiabetic medications. Obesity was defined as body mass index (BMI) ≥ 25 kg/m2, which is the proposed cutoff for the diagnosis of obesity in Asians [20]. BMI was calculated by dividing the measured weight (kg) by the square of the height (m2).
The presence or absence of fatty liver was examined through abdominal ultrasonography (US). A diagnosis of fatty liver was made on the basis of four known criteria: hepatorenal echogenic contrast, liver brightness, deep attenuation, and vascular blurring [21]. Abdominal US was performed using a 3.5-MHz transducer (Logiq 9, General Electric, Madison, WI, USA) by experienced radiologists who were unaware of the study aims and blinded to clinical information.
Colonoscopy and histologic examination
All colonoscopies were performed by experienced board-certified endoscopists, using an EVIS Lucera CV-260 colonoscope (Olympus Medical Systems, Tokyo, Japan). All participants drank 4 L of polyethylene glycol solution for bowel preparation.
All detected polypoid lesions were biopsied or removed and histologically assessed by experienced pathologists. Polyps were classified by number, size, and histologic characteristics. Pathologic results of hyperplastic polyps, inflammatory polyps, or lipomas were considered normal findings. Advanced adenoma was defined as the presence of one of the following features: > 10 mm diameter, tubulovillous or villous structure, and high-grade dysplasia (HGD) [22]. A high-risk adenoma was defined as an advanced adenoma or 3 or more adenomas [22].
Statistical analysis
Data were expressed as mean ± standard deviation or frequency (%). Age at menarche was categorized into the following groups: 10 to 11, 12 to 13, 14 to 15, 16 to 17, and 18 to 19 years old. Baseline characteristics according to age at menarche were evaluated using linear-by-linear association tests for categorical variables and by one-way analysis of variance for continuous variables.
A multivariate logistic regression analysis was performed to determine the association of any adenoma and advanced adenoma with reproductive factors including age at menarche. We estimated the adjusted odds ratio (AOR) with 95% confidence intervals (CIs) for the association of reproductive factors with the risks of any adenoma and advanced adenoma after adjusting for potential confounding variables, including age at menarche, parity, use of female hormones, menopause, smoking status, family history of CRC, history of colon polyp, NSAID use, obesity, fatty liver, hypertension, diabetes, and age. All of the reported p values are two-tailed, and p values < 0.05 were considered to be statistically significant. SPSS version 21 (IBM Co., Armonk, NY, USA) was used for statistical analyses.
RESULTS
Baseline characteristics of the study population
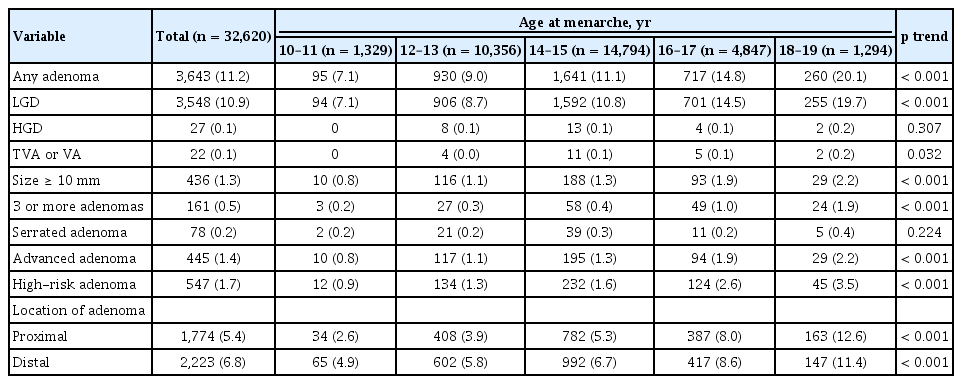
A total of 32,620 female subjects were eligible for the analysis (Fig. 1). The proportion of women with menarche at 10 to 11, 12 to 13, 14 to 15, 16 to 17, and 18 to 19 years of age was 4.1%, 31.7%, 45.4%, 14.9%, and 4.0%, respectively. Baseline characteristics of the participants stratified by age at menarche are summarized in Table 1. The mean age of the study population was 43.0 years old, and the proportion of participants aged 30 to 39, 40 to 49, and ≥ 50 years old was 42.1%, 35.1%, and 22.8%, respectively. Subjects with a younger age at menarche were more likely to be younger, and subjects with an older age at menarche were more likely to be older at baseline. The prevalence of parity, use of female hormones, and menopause was 88.7%, 2.3%, and 22.6%, respectively, and the proportions of participants with these characteristics increased in a linear manner with increasing age at menarche. Among participants with parity, the proportion of participants who had given birth more than three times increased in a linear manner with increasing age at menarche, whereas the proportion of participants who had given birth only once decreased in a linear manner with increasing age at menarche. The prevalence of history of colon polyp, use of NSAIDs, obesity, fatty liver, hypertension, and diabetes mellitus was positively associated with age at menarche, whereas the prevalence of smoking and family history of CRC was inversely associated with age at menarche.
Colonoscopic and histologic findings according to age at menarche
The colonoscopic and histologic findings of the study population according to age at menarche are presented in Table 2. Among the 32,620 female subjects, 3,643 (11.2%) had any adenomas and 445 (1.4%) had advanced adenomas. The prevalence of any adenoma, low-grade dysplasia, adenoma with villous component, adenoma larger than 10 mm, advanced adenoma, 3 or more adenomas, and high-risk adenoma increased significantly in a linear manner with increasing age at menarche. In addition, both the prevalence of proximal and distal colorectal adenoma increased significantly in a linear manner with increasing age at menarche. However, there was no significant difference in the prevalence of adenomas with HGD and serrated adenomas according to categories of age at menarche.
Risks of colorectal neoplasia according to age at menarche
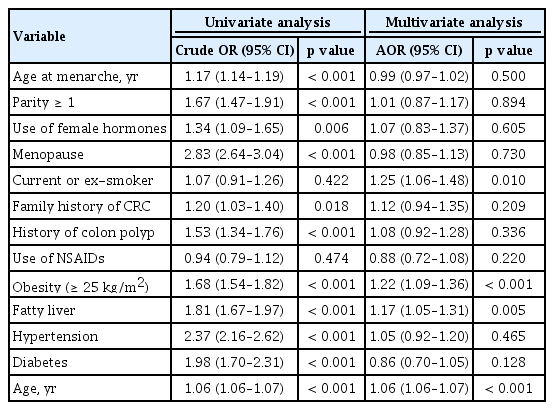
Table 3 shows the association between reproductive factors including age at menarche and risk of any adenoma. In the univariate analyses, the risk for any adenoma was significantly increased in subjects with increasing age at menarche (odds ratio [OR], 1.17; 95% CI, 1.14 to 1.19; p < 0.001). Subjects who were parous (OR, 1.67; 95% CI, 1.47 to 1.91; p < 0.001), used female hormones (OR, 1.34; 95% CI, 1.09 to 1.65; p = 0.006), were menopausal (OR, 2.83; 95% CI, 2.64 to 3.04; p < 0.001), had a family history of CRC (OR, 1.20; 95% CI, 1.03 to 1.40; p = 0.018), had a history of colon polyp (OR, 1.53; 95% CI, 1.34 to 1.76; p < 0.001), were obese (OR, 1.68; 95% CI, 1.54 to 1.82; p < 0.001), had fatty liver (OR, 1.81; 95% CI, 1.67 to 1.97; p <0.001), had hypertension (OR, 2.37; 95% CI, 2.16 to 2.62; p < 0.001), had diabetes (OR, 1.98; 95% CI, 1.70 to 2.31; p < 0.001), or were older (OR, 1.06; 95% CI, 1.06 to 1.07; p <0.001) had a higher risk for any adenoma. However, in the multivariate analyses adjusted for confounding factors including age at menarche, parity, use of female hormones, menopause, smoking status, family history of CRC, history of colon polyp, NSAID use, obesity, fatty liver, hypertension, diabetes, and age, the significant positive correlation between age at menarche and any adenoma disappeared (AOR, 0.99; 95% CI, 0.97 to 1.02; p = 0.500). In addition, parity, use of female hormones, and menopause were not significantly associated with risk of any adenoma after adjusting for confounding factors.
As with the risk for any adenoma, the risk for advanced adenoma significantly increased in subjects with older age at menarche in the univariate analyses (OR, 1.15; 95% CI, 1.09 to 1.21; p < 0.001). In addition, subjects who were parous, menopausal, obese, and older and subjects with fatty liver, hypertension, and diabetes had a significantly higher risk for advanced adenoma in the univariate analyses. However, the positive correlation between age at menarche and risk of advanced adenoma disappeared after adjusting for confounding factors (AOR, 0.98; 95% CI, 0.91 to 1.04; p = 0.468). Parity, use of female hormones, and menopause were also not associated with an increased risk of advanced adenomas in the multivariate analyses (Table 4).
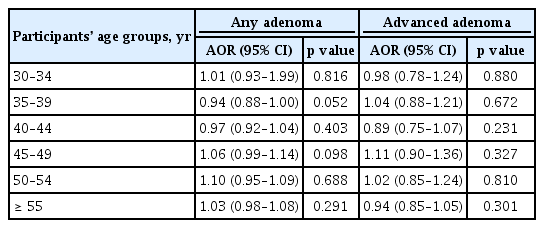
Table 5 shows the risk of colorectal adenoma according to the age at menarche among similar age groups (30 to 34, 35 to 39, 40 to 44, 45 to 49, 50 to 54, and ≥ 55 years) after adjusting for confounding factors. Age at menarche was not significantly associated with the risk of any adenoma and advanced adenoma, even in similar age groups.
We further analyzed the association between reproductive factors and risk of colorectal adenoma according to location. Reproductive factors including age at menarche were not significantly associated with risk of either proximal or distal adenoma in the multivariate analyses (data are not shown).
DISCUSSION
This large-scale study included 32,620 asymptomatic female subjects and investigated the association between age at menarche and risk of colorectal adenomas. We found that the prevalence of any adenoma and advanced adenoma increased with increasing age at menarche in univariate analysis. However, the positive association disappeared after adjusting for possible confounding factors. In other words, there was no significant association between age at menarche and risk of both any and advanced adenoma. In addition, age at menarche was not significantly associated with the risk of adenoma, even among similar age groups. Other reproductive factors such as parity, use of female hormones, and menopausal status were also not associated with the risk of adenoma in multivariate analysis.
Colorectal adenomas as well as CRC occur more frequently in men than in women [6-8]. We speculated that one reason for this may be that female hormones play a protective role in colorectal carcinogenesis. The exact biologic mechanisms for the relationship between female hormones and the risk of CRN are not fully understood, but several experimental studies support our hypothesis and have provided evidence for a protective effect of female hormones on CRC development through a variety of mechanisms. First, it has been proposed that female hormones directly regulate cell growth in the colonic epithelium and inhibit cell proliferation of colorectal tumors by binding to the estrogen receptor [11,12,23]. Second, researchers have identified estrogen receptor genes that can act as tumor suppressors in the presence of exogenous estrogen [12,24]. Third, female hormones may act indirectly to decrease secondary bile acid levels and downregulate insulin-like growth factor-1, a hormone that has been implicated in CRC pathogenesis [11,24].
Age at menarche has been used as a surrogate marker for the duration and amount of endogenous female hormone exposure in a woman’s lifetime [25,26]. Considering the protective role of female hormones on colorectal carcinogenesis described in previous studies, we would expect that women with late menarche, and thus less exposure to female hormones, have an increased risk of developing CRN [11,12]. In addition, there may be other reproductive factors that act to modify the process of carcinogenesis in the colon and affect the development of CRN.
To date, many studies have evaluated the association between reproductive factors including age at menarche and the risk of CRC [15-19]. Although the results have not been consistent, there has been some suggestion that age at menarche might be related to the risk of CRC [15,16]. Additionally, meta-analyses have demonstrated that oral contraceptive use and hormone replacement therapy may reduce the risk of CRC [27,28].
Based on previous studies on CRC, reproductive factors including age at menarche may play a role in the pathophysiologic processes associated with the development of colorectal adenomas. However, while many studies have investigated CRC risk, few have attempted to explore this relationship for colorectal adenoma risk, and thus little is known about the influence of reproductive factors on the development of colorectal adenomas [29-31]. To date, only a few studies have investigated the relationship between reproductive factors and colorectal adenomas, and these have yielded conflicting results. One study from a large prospective cohort reported no significant association between age at menarche, menopausal status, or oral contraceptive use and distal colorectal adenomas, whereas women with higher parity had an increased risk of distal colorectal adenomas [29]. However, the study did not evaluate cases with adenomas in the proximal colon [29]. A case-control study that included 347 subjects also showed a lack of association between age at menarche and colorectal adenoma risk [30]. The authors reported that parity and oral contraceptive use was not associated with adenoma risk, whereas increasing age at menopause and non-contraceptive hormone use were associated with a reduced risk of adenomas [30]. In contrast to the studies described above, another case-control study consisting of 411 subjects revealed that women with early menarche before age 13 years old had lower risk for adenomas, compared to those with late menarche, while parity, oral contraceptives and non-contraceptive hormone use were not associated with risk of adenomas [31]. However, the aforementioned case-control studies had a small number of study participants and thus provided insufficient evidence from which to draw definite conclusions about this issue.
Contrary to our expectations, age at menarche did not influence the risk of either any or advanced adenoma development. Other reproductive factors including parity, use of female hormones, and menopausal status were also not associated with the risk of adenomas. Our results suggest that reproductive factors including age at menarche may not affect the development of colorectal adenomas. Our findings seem to be in line with those of a recent meta-analysis of 11 case-control and 11 cohort studies that explored the association between age at menarche and CRC risk, and found no association between the two variables [13]. Nevertheless, it would be premature to conclude that female hormones have no protective effect on the development of CRN based on the results of the reproductive factors analyzed in this study. It is possible that other factors related to female hormone levels may influence CRN risk. The effect of sex differences on the prevalence of CRN remains unresolved and further studies are needed to elucidate this issue.
Interestingly, in multivariate analysis adjusted for confounding factors, a significant correlation between current or ex-smoker status and colorectal adenoma was observed despite the absence of significance in univariate analysis. In sensitivity analysis, current or ex-smoker status became a significant risk factor for colorectal adenoma when age was adjusted. These results mean that smoking status was influenced by age and thus, when age was adjusted, the significant association between current or ex-smoker and colorectal adenoma appeared.
Our study included a large number of asymptomatic female subjects who underwent complete colonoscopy, and thus may show more reliable results than previous studies. Nevertheless, there are several limitations to the present study. First, this was a retrospective study with a corresponding potential bias in design. However, we think that there was minimal selection bias, because our cohort was established prospectively. Second, our study was hospital-based rather than population-based and our cohort was recruited at two medical examination centers in Korea. Therefore, interpretation of our findings requires careful consideration, because the extent to which our findings are generalizable to populations with different sociodemographic characteristics and ethnicities is unclear. Third, we did not investigate the duration between menarche and menopause, which may be a more reliable indicator of the degree of exposure to female hormones. However, in our study, a large proportion of study participants were premenopausal women aged < 50 years old and the proportion of postmenopausal women was only 22.6%. It may be inappropriate to conclude that among premenopausal women, the duration of exposure to female hormones is the duration between age at menarche and current age. Fourth, we did not investigate the duration of oral contraceptive use, hormone replacement therapy, and breast feeding, which may be important factors for determining the degree of exposure to female hormones. Fifth, the great majority of study participants were younger than 50 years old and the proportion of subjects aged 30 to 39 was not low (42%). Therefore, there may be some limitation with respect to evaluating reproductive factors such as parity and use of female hormones. Finally, the current study included patients with previous colonoscopy or history of colon polyp. This may affect the association between age at menarche and risk of colorectal adenomas, although a history of colon polyp was adjusted as a confounding factor in multivariate analysis.
In conclusion, age at menarche was not significantly associated with the risk of colorectal adenomas. In addition, parity, use of exogenous female hormones, and menopausal status were not associated with the risk of colorectal adenomas. Our results suggest that reproductive factors including age at menarche may not affect the development of colorectal adenomas.
KEY MESSAGE
1. There was no significant association between age at menarche and risk of any or advanced adenoma.
2. Age at menarche was not significantly associated with the risk of adenoma, even among similar age groups.
3. Other reproductive factors such as parity, use of female hormones, and menopausal status were also not associated with the risk of any or advanced adenoma.
Notes
No potential conflict of interest relevant to this article was reported.