Efficacy and safety of cisplatin and weekly docetaxel in patients with recurrent or metastatic squamous cell carcinoma of the head and neck
Article information
Abstract
Background/Aims
We investigated the efficacy and toxicity of a weekly schedule of docetaxel and cisplatin as a first-line treatment in patients with recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC).
Methods
In this study, 18 patients with previously diagnosed R/M HNSCC were treated with combination chemotherapy of weekly docetaxel 35 mg/m2 (day 1 and 8) and cisplatin 70 mg/m2 (day 1) as first-line chemotherapy, repeated every 3 weeks.
Results
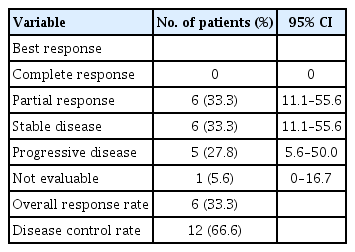
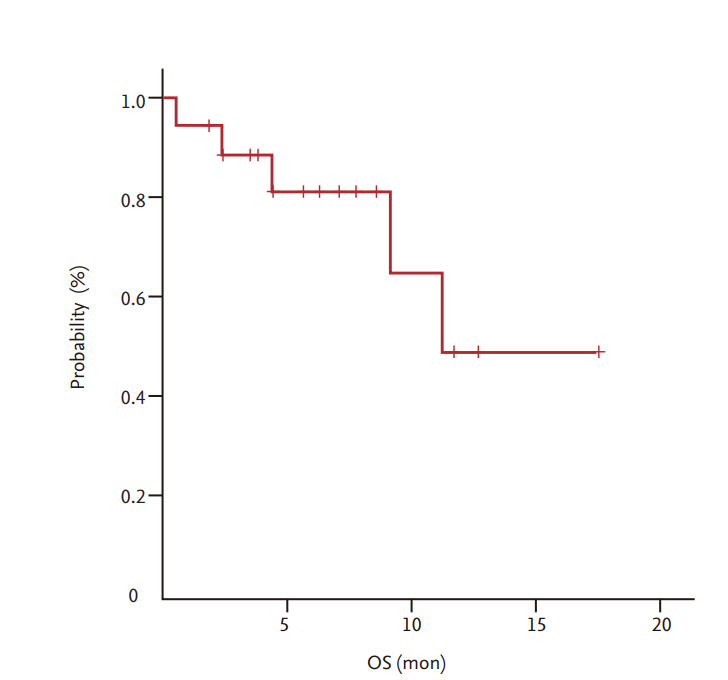
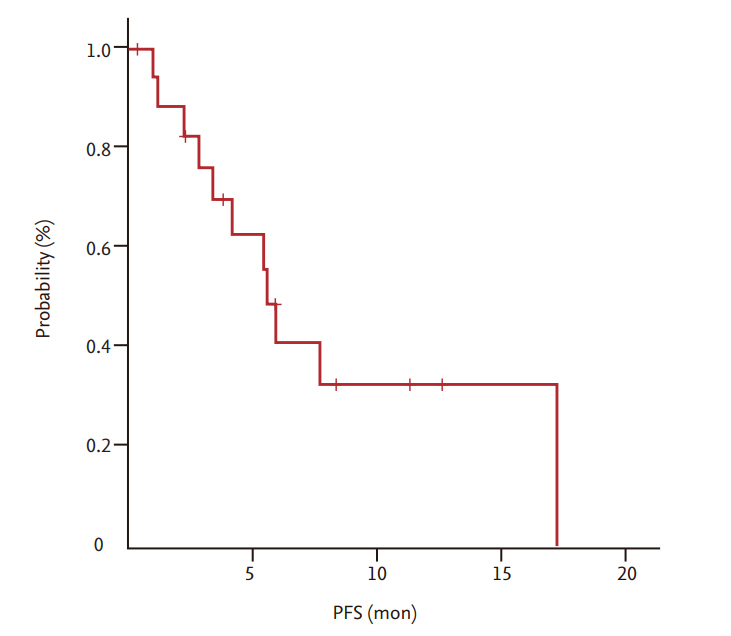
Partial response and stable disease were observed in six patients (33.3%; 95% confidence interval [CI], 11.1% to 55.6%) and six patients (33.3%; 95% CI, 11.1% to 55.6%), respectively. The median overall survival and progression-free survival were 11.26 months (95% CI, 8.87 to 15.83) and 5.68 months (95% CI, 4.80 to 6.51), respectively. The major toxicity was grade 1/2 anemia (50%). Grade 3/4 neutropenia was observed in one patient (5.6%). Among the non-hematologic toxicities, grade 1/2 hepatotoxicity was most common (22.2%), and grade 3/4 infection was observed in one patient (5.6%). There was no treatment-related mortality.
Conclusions
For patients with R/M HNSCC, a cisplatin and weekly docetaxel regimen showed high efficacy with tolerable toxicity as a first-line treatment.
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide, with approximately 500,000 new cases diagnosed annually [1]. Most patients with HNSCC present with locally or regionally advanced disease that requires concurrent chemotherapy and radiotherapy or surgery [2,3]. However, approximately 10% of patients with HNSCC have distant metastasis at the time of initial presentation, and up to 20% of patients experience relapse in a site that requires chemotherapy. For these patients with recurrent or metastatic (R/M) HNSCC, platinum-based palliative chemotherapy is commonly used for first-line treatment, resulting in a median survival of 6 to 9 months and an 1-year survival rate of 40% [4].
Docetaxel, a semisynthetic taxoid derived from the rare Pacific yew tree Taxus baccata [5], is active against R/M HNSCC. The combination of docetaxel and platinum-based cytotoxic agents has led to a high response rate, ranging from 33% to 53%, in previous studies [6-9]. However, owing to severe myelosuppression related to the 3-week dosing schedule of docetaxel, an alternative schedule of weekly docetaxel as a single agent has been evaluated in several studies, and showed comparable efficacy and acceptable toxicity in several cancers [10-14]. We previously reported that this combination regimen has an acceptable outcome as first-line chemotherapy in patients with recurrent or metastatic nasopharyngeal cancer, with an objective response rate (ORR) of 70.2% and a median overall survival (OS) of 28.5 months [14]. Kim et al. [15] also showed that a regimen of weekly docetaxel and cisplatin was active and tolerable as front-line treatment in advanced gastric cancer. In contrast, studies on the efficacy and toxicity of a platinum doublet with a weekly schedule of docetaxel have not yet been reported in R/N HNSCC.
Therefore, we designed this single-center, retrospective study to evaluate the efficacy and toxicity of cisplatin and weekly docetaxel as first-line chemotherapy in the treatment of R/M HNSCC.
METHODS
Patient eligibility
We retrospectively collected and reviewed the medical records of patients with cytologically or histologically confirmed HNSCC. Other eligibility criteria included at least one measurable lesion according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.0; age > 18 years; Eastern Cooperative Oncology Group (ECOG) performance status 0–2; and adequate hematologic function with absolute neutrophil count (ANC) > 1,500/mm3, platelet count > 100,000/mm3; adequate liver function with total bilirubin < 1.5 mg/dL, aspartate aminotransferase, alanine aminotransferase < 2.5 × upper limit of normal (ULN), alkaline phosphatase < 5 × ULN in patients without liver metastasis; and adequate renal function with serum creatinine < 1.5 mg/dL or calculated creatinine clearance > 60 by the Cockcroft-Gault method. Prior chemotherapy for recurrent or metastatic disease was not permitted. Patients with prior induction or adjuvant chemotherapy were eligible if at least 6 months had elapsed from their last course of chemotherapy. Other exclusion criteria included active infection, history of myocardial infarction in the last 3 months, uncontrolled congestive heart failure or hypertension, uncontrolled diabetes mellitus, history of prior chemotherapy with docetaxel, pregnancy, lactation, and other malignancy within 5 years. This study was approved by the Institutional Review Board of Samsung Medical Center (2014-02-049). All patients were drawn from existing research databases and had provided informed consent to be included in research studies on these treatments.
Treatment
Cisplatin was administered at a dose of 70 mg/m2 by intravenous infusion in 150 mL normal saline over 60 minutes on day 1, and docetaxel was administered a dose of 35 mg/m2 by intravenous infusion in 100 mL dextrose water over 60 minutes on days 1 and 8. All patients received adequate hydration, with at least 1,500 mL of half-normal saline or normal saline prior to cisplatin administration. Patients also received antiemetic therapy consisting of an intravenous 5-HT3 antagonist and dexamethasone before docetaxel administration. The treatment cycles were repeated every 3 weeks for a maximum of six cycles.
Toxicities were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. Patients whose absolute neutrophil and platelet counts were ≥ 1,500 and 100,000/mm3, respectively, and who had grade 1 or less non-hematologic toxicity (excluding alopecia), received chemotherapy on day 1 of each cycle.
On days 1 and 8 of each cycle, the minimum requirements to receive chemotherapy were an ANC between 1,000 and 1,500/mm3, a platelet count ≥ 75,000/mm3 and no grade ≥ a non-hematologic toxicity (excluding alopecia). If there were any grade 3 or 4 hematologic toxicities at the nadir of the previous cycle, or febrile neutropenia with or without documented infection, then administration of both drugs in the subsequent cycles was reduced 20% from the planned dose. The administration of granulocyte-colony stimulating factor was allowed in the presence of febrile neutropenia or grade 3 or 4 neutropenia. In the presence of grade 3 or 4 non-hematologic toxicity (except nausea, vomiting, and alopecia), the treatment was postponed until resolution of the toxicity and then both drug doses were reduced by 25% for the next cycle. In the case of grade 2 neurotoxicity, treatment was postponed until resolution of the toxicity to the level of grade 1 or less within 3 weeks; at that point, the doses of both drugs in subsequent cycles were reduced by 25% from the planned dose. In the presence of grade 2 asthenia, the subsequent cycle was also postponed until resolution to the level of grade 1 or less within 3 weeks; subsequent cycles were then performed with a 25% dose reduction from the planned dose of docetaxel. Treatment was stopped at any time for documented disease progression or unacceptable toxicity, or at the patient’s request.
Assessment of efficacy
The pretreatment baseline evaluation included a complete medical history and physical examination, complete blood cell count (CBC) with differential, chemistry profiles, and performance status. Chest radiography, chest and upper abdominal computed tomography (CT) imaging, brain CT or magnetic resonance imaging (MRI), radionuclide bone scans, and other diagnostic procedures were performed as clinically indicated.
During treatment, a limited history, physical examination, assessment of toxicity, CBC with differential, and blood chemistry tests were repeated weekly. A chest radiograph was obtained every 3 weeks, prior to each cycle. Appropriate imaging studies, including CT scans of the chest and upper abdomen, were performed every two cycles to assess the treatment response, and sooner if required to document disease progression. Objective tumor responses were assessed according to the RECIST criteria using CT scan or MRI [16].
Statistical analysis
SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The response rate was calculated as the ratio of the number of patients who achieved a complete response (CR) or partial response (PR) to the number of enrolled patients. Progression-free survival (PFS) was defined as the time from the date of first administration of chemotherapy to the date of documented progression or loss to follow-up or death due to any cause. OS was defined as the time from the date of first administration of chemotherapy to the date of death or final follow-up. Response rate and patient clinical characteristics were evaluated using the chi-square test or Fisher’s exact test. PFS and OS were calculated with the Kaplan-Meier method and the 95% confidence interval (CI) for the median time to an event.
RESULTS
Patient characteristics
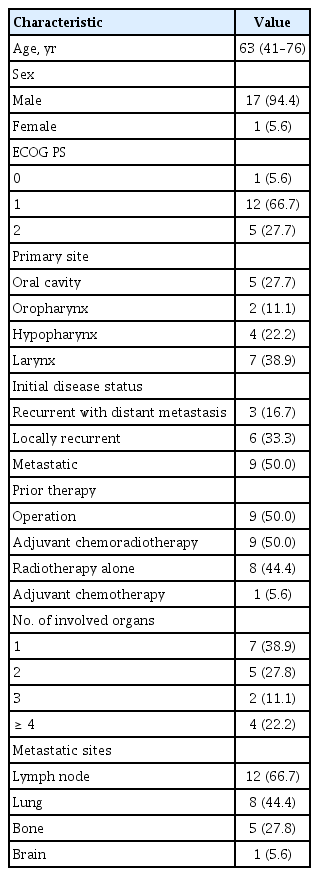
Between August 2007 and October 2013, a total of 18 patients were enrolled from a single center, Samsung Medical Center. Baseline patient characteristics are shown in Table 1. All 18 patients enrolled in the study, comprising 17 males (94.4%) and one female (5.6%), were included in an intent-to-treat analysis for the safety profile. One patient’s response could not be evaluated owing to loss to follow-up after one cycle of chemotherapy. The median age of the patients was 63 years (range, 41 to 76). Seven patients (38.9%) were older than 65 years. All 18 patients were former or current smokers. Subsequent chemotherapy was given to five patients who experienced progression following the study treatment. Only two patients received more than second-line chemotherapy. The most common site of metastasis was the lymph nodes (n = 12), followed by lung (n = 8), bone (n = 5), and brain (n = 1).
Treatment administration
A total of 61 cycles of chemotherapy were administered, with a median number of three cycles per patient (range, 1 to 6). Two patients (11.1%) completed six cycles of chemotherapy as planned, two (11.1%) received five cycles, four (22.2%) received four cycles, and 10 (55.6%) received fewer than four cycles. Dose reduction was required in two patients, and administration of docetaxel on day 8 was omitted in two patients. The main cause of dose reduction was grade 3 to 4 neutropenia.
Treatment response and survival rate
Among a total of 18 patients, 17 were evaluated for treatment response. In one patient, early discontinuation of the study occurred because of loss to follow-up before the first evaluation. As shown in Table 2, the ORR (CR + PR) was 33.3% (6/18) and all six patients achieved PR 33.3% (95% CI, 11.1% to 55.6%). Six patients (33.3%) were confirmed to have stable disease. With a median follow-up duration of 26.8 months (range, 8 to 70.5), the median OS and PFS were 11.26 months (95% CI, 8.87 to 15.83) (Fig. 1) and 5.68 months (95% CI, 4.80 to 6.51) (Fig. 2), respectively.
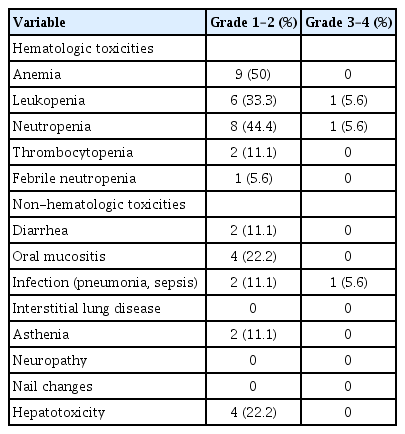
Toxicity
All patients were evaluated for toxicity. Table 3 shows the major hematologic and non-hematologic toxicities. Grade 1/2 anemia was the most common toxicity (50%). Grade 3/4 febrile neutropenia occurred in one patient (5.6%), who experienced grade 4 neutropenia. Grade 3/4 neutropenia was observed in one patient (5.6%). Among the non-hematologic toxicities, grade 1/2 hepatotoxicity was the most common (22.2%), and grade 3/4 infection was observed in one patient (5.6%). There was no treatment-related mortality.
DISCUSSION
In the present study, the combination of cisplatin and weekly docetaxel chemotherapy as first-line treatment of R/M HNSCC showed high efficacy, with a 33.3% response rate and tolerable toxicity. These results are consistent with the findings of previous studies demonstrating a response rate of 33% to 53%. It is of note that grade 1/2 anemia was the major toxicity, observed in 50% of patients, but grade 3/4 neutropenia was only found in one patient. Moreover, the median PFS was 5.6 months, and the median OS was 11.26 months, which is a quite encouraging finding.
Previously, a number of chemotherapeutic agents including platinum, taxanes, 5-fluorouracil (5-FU), methotrexate, and ifosfamide have been demonstrated to have single-agent activity as first-line treatment for patients with R/M HNSCC [17,18]. In 1992, two studies established the use of platinum-based doublets with 5-FU in R/M HNSCC by showing a higher response rate (21% to 32%) than that achieved with monotherapy (17%), without survival gain [19,20]. Other chemotherapy doublets have also been evaluated as first-line treatment of R/M HNSCC [6-9,21-27]. Among them, a 3-week schedule of combined docetaxel and cisplatin was found to have remarkable efficacy despite its severe toxicities [6-9]. Glisson et al. [8] (n = 36) reported an ORR of 40% (6% CR, 34% PR), a median PFS of 4 months, and an OS of 9.6 months for a triweekly docetaxel 75 mg/m2 and cisplatin 75 mg/m2 regimen. However, grade 4 neutropenia was observed in 71% of patients. Other grade 3 and 4 side effects included asthenia (25%), nausea (11%), fever (8%), and diarrhea (8%). Caponigro et al. [9] (n = 46) demonstrated an ORR of 46% (11% CR, 35% PR) and a median OS of 11 months for a 3-week docetaxel 75 mg/m2 and cisplatin 100 mg/m2 regimen. However, a high incidence of grade 3–4 neutropenia (61%) was also noted in this study. Given the high incidence of poor performance status, the high prevalence of comorbid conditions, and risk factors including smoking and alcoholism in patients with R/M HNSCC, chemotherapeutic agents with less toxicity should be developed. In this context, our regimen of cisplatin with weekly docetaxel is reasonable in terms of efficacy and toxicity.
It has been reported that East Asian ethnic groups experience more severe toxicities including myelosuppression or mucositis with a 3-week schedule of docetaxel compared to Western ethnic groups [28-31]. This phenomenon is thought to be the result of pharmacogenomic differences between the two ethnic groups. According to previous studies, polymorphisms in cytochrome P450, family 3, subfamily A (CYP3A) isoenzymes, which inactivate docetaxel, are believed to play an important role in these pharmacokinetic differences. In East Asian patients, CYP3A activity and docetaxel clearance are notably lower than those in Western patients [28-31]. Docetaxel causes characteristic early bone marrow suppression, which occurs 5 to 9 days after infusion, whereas this adverse effect appears 10 to 14 days after cisplatin infusion; thus, we hypothesized that splitting the docetaxel schedule would reduce overlapping toxicities compared to the 3-week schedule of docetaxel and cisplatin [32].
Our group previously reported a very high response rate (70%) and low hematologic or non-hematologic toxicities (no grade 3/4 toxicity) with this combination regimen in patients with recurrent or metastatic nasopharyngeal cancer, even though the nature of the disease and patient characteristics are different [15]. Several studies regarding a regimen of weekly docetaxel as a single agent in the treatment of R/M HNSCC have demonstrated an excellent toxicity profile, particularly notable for a low rate of grade 3/4 neutropenia, ranging from 0% to 12.5%, but a variable ORR (7% to 42%) and OS (17.9 weeks to 11.3 months) [33-36]. Three [33,35,36] of four studies showed suboptimal response rate and survival, but these differences could be explained by differences in the study populations, which included patients who received palliative chemotherapy as second-line or more for R/M HNSCC. Okamoto et al. [33] compared the efficacy and safety of weekly docetaxel at a dose of 25 to 30 mg/m2 with a triweekly docetaxel regimen of 60 mg/m2. The ORR was 22% for the 18 patients (one pretreated) in the weekly docetaxel group, and 47.8% for the 29 patients (10 pretreated) in the triweekly docetaxel group. Only one (5.6%) case of grade 3 mucositis developed in the group receiving weekly docetaxel, while 12 cases of grade 3 or 4 neutropenia (41.4) and two cases of grade 3 or 4 thrombocytopenia (6.9%) occurred in the triweekly docetaxel group. Cho et al. [35] evaluated the efficacy and toxicity of weekly docetaxel at a dose of 35 mg/m2 in patients with platinum-refractory R/M HNSCC and obtained an acceptable clinical outcome, with an ORR of 13% and an OS of 29 weeks (95% CI, 10.8 to 47.1) in the setting of second-line or further chemotherapy. Grade 3 neutropenia occurred in only 8.7% of patients. Specenier et al. [36] reported the lowest ORR of the studies cited, at 7%, along with an OS of 17.9 weeks (95% CI, 10.1 to 25.6), in 30 patients receiving weekly docetaxel of 36 mg/m2, among whom 27 (90%) were pretreated patients. There were no reported episodes of grade 4 neutropenia, thrombocytopenia, or non-hematological toxicity.
To the best of our knowledge, this is the first study to investigate the efficacy and acceptable toxicities of cisplatin doublets with weekly docetaxel as first-line treatment for patients with R/M HNSCC in Asia. Considering the small number of patients enrolled in this study, large randomized pharmacogenomic and epidemiologic studies would be helpful in establishing the basis of differences in toxicity and efficacy among patients with R/M HNSCC.
The present study has some limitations. First, this study was performed at a single institution with a small study population. Thus, a larger number of patients along with a comparative study are needed to confirm our results. Secondly, no information was available about tumor human papilloma virus (HPV) status, which may have contributed to heterogeneity in prognosis within the patient population. The prognostic impact of HPV on HNSCC has been evaluated in numerous studies over the past two decades, and HPV-positive status is widely known to be associated with a better prognosis [37-46]. In addition, the results of four phase III clinical trials have confirmed HPV tumor status as a predictor of prognosis regardless of treatment regimen [37-40].
In conclusion, the present study demonstrated that a regimen of cisplatin and weekly docetaxel was clinically efficacious and had tolerable toxicities as first-line chemotherapy in patients with R/M HNSCC.
KEY MESSAGE
1. The standard treatment for locally advanced head and neck squamous cell carcinoma varies worldwide.
2. For patients with recurrent, metastatic recurrent, or metastatic squamous cell carcinoma of the head and neck, a regimen of cisplatin and weekly docetaxel showed high efficacy with tolerable toxicity as a first-line treatment.
Notes
No potential conflict of interest relevant to this article was reported.