Hepatitis B Reactivation During Adjuvant Anthracycline-Based Chemotherapy in Patients with Breast Cancer: A Single Institution's Experience
Article information
Abstract
Background
The objectives of this study were to determine the incidence, outcome and risk factors for HBV reactivation in HBsAg positive breast cancer patients while on anthracycline -based adjuvant chemotherapy.
Methods
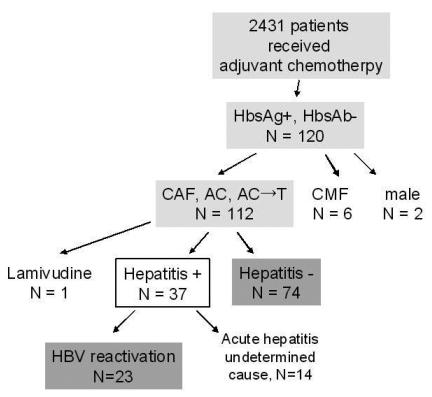
We retrospectively reviewed the records of 2,431 patients with early breast cancer who received adjuvant chemotherapy from March 2001 to December 2005. Among these patients, 111 HBsAg positive women were enrolled in this study.
Results
Thirty-seven patients (33.3%) developed acute hepatitis, of which 23 (20.7%) were related to HBV reactivation. Univariate analysis showed that an age ≥47 years (p=0.034) and abnormal sonographic findings such as a fatty liver or cirrhotic changes (p=0.034) were associated with HBV reactivation. However, an HBeAg positive status and the use of corticosteroids were not. Multivariate analysis found that no clinical factors could predict HBV reactivation during chemotherapy. All 23 patients who developed HBV reactivation received lamivudine as a therapeutic measure at the time of HBV reactivation. Despite the use of lamivudine, disruption in the chemotherapy protocol occurred in 18 patients (78.3%) and 14 of these patients had premature termination of their chemotherapy.
Conclusions
HBV reactivation occurred in a significant proportion of HBsAg positive patients during adjuvant anthracycline-based chemotherapy. Once hepatitis developed, most patients could not finish the chemotherapy as planned despite lamivudine treatment. Until the risk factors for reactivation are clearly identified, HbsAg-positive patients should begin prophylactic antiviral treatment before initiating chemotherapy.
INTRODUCTION
Korea is recognized as an endemic area of hepatitis B virus (HBV) infection, with an HBsAg prevalence of 5.1% in men and 4.1% in women1). HBV reactivation frequently occurs in cancer patients undergoing chemotherapy who are HBV carriers2-4). HBV reactivation is characterized by an increased serum HBV DNA level, abnormal liver function tests and clinical hepatitis. The severity of the condition ranges from anicteric hepatitis, which may recover spontaneously, to fatal hepatic failure3, 5).
The mechanism of HBV reactivation during chemotherapy may be associated with the suppression of the normal immunological responses to HBV, leading to enhanced viral replication and widespread infection of hepatocytes6, 7). When the cancer chemotherapy is discontinued, immune competence is restored and infected hepatocytes are rapidly destroyed.
In reports from China and Greece, the risk of chemotherapy-induced HBV reactivation was reported to range from 14% to 72%8-11). Lamivudine, a cyclic nucleoside analogue, is effective in suppressing HBV DNA, normalizing liver enzymes and improving the histology in both HBeAg-positive and -negative/HBV-DNA positive patients. This effect suggests that pophylactic antiviral therapy might reduce the risk of HBV reactivation and prevent the associated complications12, 13). However, these previous studies, were limited by their small sample size, heterogeneity in the tumor type and varying chemotherapy regimens10, 11). The immunosuppressive effects of adjuvant chemotherapy for the treatment of patients with breast cancer are not considered severe14). The optimal duration of prophylactic lamivudine therapy is uncertain. Use of this medication must take into consideration the potential emergence of the lamivudine- resistant HBV strain, the so-called YMDD mutation that can develop during lamivudine prophylaxis15, 16). Currently, the indications for prophylactic therapy, to prevent HBV reactivation in patients receiving adjuvant chemotherapy, are not clear17).
Although Korea is an endemic area for chronic HBV infection, there have been few clinical studies on the reactivation of HBV during treatment with chemotherapy for solid tumors. We therefore retrospectively assessed the incidence of HBV reactivation and hepatitis in HBsAg positive patients who were receiving breast adjuvant chemotherapy to identify the risk factors associated with HBV reactivation.
MATERIALS AND METHODS
Patients
A review of the Breast Cancer Adjuvant Treatment Registry at the Asan Medical Center revealed that 2,431 patients received adjuvant chemotherapy from August 2001 through December 2005. Among these patients, we selected those who (1) were serologically HBsAg positive before adjuvant chemotherapy; (2) received anthracycline-based chemotherapy and (3) had adequate liver function (liver transaminase levels ≤3 times the upper normal limit and serum bilirubin ≤1.5 mg/dL). We excluded patients who had previously received anti-HBV therapy (e.g. lamivudine or interferon alpha) and those with decompensated liver function at screening (prolonged prothrombin time, history of ascites, hypoalbuminemia, history of ascites, variceal bleeding, or hepatic encephalopathy). Of the 2,431 patients, 120 were HBsAg seropositive. After excluding nine patients (2 men, 1 woman who was receiving lamivudine and 6 women who received CMF regimens), 111 patients were included in this study (Figure 1).
Laboratory studies and monitoring for HBV reactivation
According to the standard admission protocol of our surgical department, breast cancer patients eligible for surgery were screened for hepatitis-B serology (i.e.HBs Ag, anti-HBs and antiHBc IgG) using a commercially available immunoassay (A6K-3® RIA-IRMA Immuno-RadioMetric Assay; Diasorin Inc., Stillwater, MN, USA). In addition, their serum protein, albumin, bilirubin, alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT)) and clotting profile were evaluated, and they were tested for the HCV antibody. The HBeAg, anti-HBe, antiHBc IgM, and serum HBV DNA level were also studied in patients who were HBsAg positive. The serum HBV DNA was quantitated using the hybrid capture assay (Digene Corp., Gaithersburg, MD, USA), which has a limit of detection of 1.4×105 copies/mL (1pg HBV DNA=283,000 copies (~3×105 viral genome equivalents))18). Ultrasonographic examination of the liver was performed within 2 weeks prior to surgery.
On day 1 and day 8 of the first cycle of adjuvant chemotherapy, before each subsequent cycle and 4 weeks after the completion of chemotherapy, all patients were monitored by testing for the complete blood count, renal function tests and liver function tests, and assessment of their clinical signs and symptoms. When a patient was found to have developed hepatitis (as defined below) during chemotherapy, HBeAg, anti-HBe and HBV DNA were measured, along with tests for hepatitis A, hepatitis C and the antinuclear factor.
Adjuvant chemotherapy
Patients were treated with anthracycline-based chemotherapy regimens, either AC (adriamycin 60 mg/m2 and cyclophosphamide 600 mg/m2 iv push on day 1 for four cycles), CAF (cyclophosphamide 600 mg/m2, adriamycin 60 mg/m2 and 5FU 600 mg/m2 iv push on day 1 for six cycles), or four cycles of AC followed by four cycles of paclitaxel 175 mg/m2 iv infusion. Patients with ≥5 cm sized primary tumors or≥4 positive nodes, and patients who underwent breast-conserving operations were treated with adjuvant radiotherapy after chemotherapy. Patients with ER or PR positive tumors were treated for 5 years with tamoxifen therapy after adjuvant chemotherapy.
Definitions of hepatitis and HBV reactivation
The severity of hepatic dysfunction was defined as grade 1 when ALT was ≤2.5 X upper limit of normal (ULN); grade 2 when ALT was between 2.5 X ULN and 5 X ULN; grade 3 when ALT was between 5 X ULN and 20 X ULN; and grade 4 when ALT was > 20 X ULN. Hepatitis was defined as ALT ≥3 X ULN or a > 100U/L increase over baseline (3). Hepatitis attributable to HBV reactivation was defined as a positive seroconversion from negative (serum HBV DNA < 1.4×105 copies/mL) or increase of serum HBV DNA level ≥2 X baseline in addition to the development of hepatitis as defined above19). Icteric hepatitis was defined as hepatitis accompanied by a serum bilirubin two-fold higher than the reference range (< 1.2 mg/dL). Disruption of chemotherapy treatment was defined as either premature termination or a delay of more than 8 days between cycles.
Statistical analysis and risk factor assessment
Among the risk factors assessed were patient age (younger or older than median age), HBeAg status, pattern of liver ultrasonography (e.g. fatty liver/cirrhosis), baseline ALT level, chemotherapeutic regimen, and use of corticosteroids during chemotherapy. Association of these factors with the development of HBV reactivation was determined using the 2 test or Fisher's exact test, as appropriate. Two-tailed p-values of ≤ 0.05 were regarded as significant. All calculations were performed using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Baseline characteristics
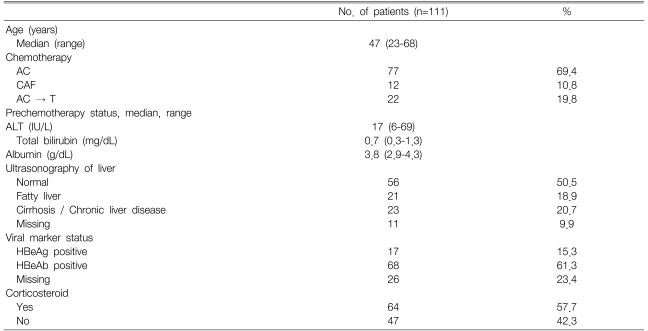
All 111 patients were women; their median age was 47 years (range, 23-68 years), and they received a median of five chemotherapy cycles (range, 1 to 8 cycles). Of the 111 HBsAg seropositive patients, 77 (69.4%) received the AC (adriamycin/ cyclophosphamide) regimen, 22 patients (19.8%) received AC followed by T (paclitaxel) chemotherapy, and 12 (10.8%) received the CAF (cyclophosphamide/adriamycin/fluorouracil) regimen. Five patients had grade 1 hepatic dysfunction at baseline and all others had normal liver function. Fifty-six patients showed normal patterns on hepatic ultrasonography, whereas 44 (39.6%) had abnormal sonographic features (fatty liver or cirrhotic change). Seventeen patients (15.3%) were HBeAg seropositive. Sixty-four patients (55.7%) were treated with corticosteroids for its antiemetic effect and as a paclitaxel premedication (Table 1).
Hepatitis due to HBV exacerbation
Sixty-two patients (60.4%) with normal baseline liver function experienced one or more episodes of grade 1 hepatic dysfunction during adjuvant chemotherapy. Thirty-seven patients (33.3%) developed acute hepatitis, with 23 (20.7%) considered to be due to HBV reactivation. Of these 23, 12 patients (52.2%) developed icteric hepatitis. The severity of hepatitis attributable to HBV reactivation was grade 2 in 1 patient, grade 3 in 9 patients and grade 4 in 13 patients. The time from the identification of an abnormal (grade 1) liver function test to the detection of HBV DNA was a median of 22 days (range, 0-29). The median number of cycles of chemotherapy administered before the detection of hepatitis was three (range, 2-6). Seven patients had HBV reactivation right after the last cycle of planned chemotherapy. The median HBV DNA level in these 23 patients was 2.1×107 copies/mL (Table 2). Lamivudine treatment was initiated after a median of 7 days from the detection of hepatitis (range, 0-25 days).
Hepatitis due to undetermined causes
Fourteen patients developed hepatitis from unknown causes; the HBV DNA was not detected in any of these patients. In all 14 patients, the tests for the diagnosis of hepatitis A and C were negative. One patient had a history of taking Ginseng and another had taken acetaminophen for 7 days. The severity of hepatitis was grade 2 in 6 patients, grade 3 in 4 patients, and grade 4 in 4 patients. None of these patients had icteric hepatitis. These patients received a median of 4 cycles of chemotherapy (range, 1-5 cycles) before the detection of hepatitis.
Clinical outcomes and impact on chemotherapy
The 23 patients who developed acute hepatitis related to HBV reactivation had a baseline ALT level before chemotherapy of 18 IU/L (range 7-38 IU/L). Their peak ALT while on chemotherapy was 584 IU/L (range 127-2362 IU/L). Sixteen patients (70%) were hospitalized for a median 11 days (range 5-40 days). All 23 patients were treated with lamivudine for a median of 209 days (range 52 to 385 days). The hepatic dysfunction was normalized within a median 39 days (range, 7-162 days) in all patients. However, the chemotherapy was disrupted in 18 patients (78.3%); the chemotherapy was delayed in 4 patients (17.4%) and terminated early in 14 (60.9%) (Table 2). In patients who had their chemotherapy terminated early, the hepatic dysfunction normalized within a median of 51 days (range, 23-162 days). Among the 14 patients who had acute hepatitis of unknown cause, only one patient was hospitalized for 5 days, whereas disruption of the chemotherapy occurred in five patients.
Risk factor assessments
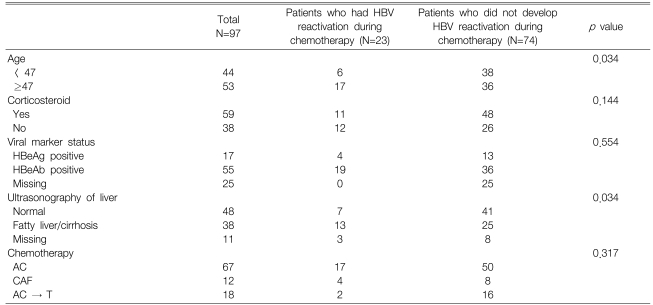
Univariate analysis showed that, patients ≥47 years old (p=0.034) and those with fatty liver or cirrhotic changes on hepatic ultrasonography (p=0.034) were at greater risk for the development of hepatitis related to HBV reactivation (Table 3). However, neither HBeAg positivity nor the use of corticosteroids was significantly correlated with HBV reactivation. The multivariate analysis identified no clinical factor that could predict the development of HBV reactivation.
DISCUSSION
HBV reactivation is common in patients with malignancies, especially during chemotherapy. Although prophylactic lamivudine before chemotherapy has been recommended for patients with hematological malignancies, little is known about the frequency, risk factors and prophylactic use of lamivudine for HBV reactivation in patients with solid tumor cancers8, 9, 12). Several studies in patients with solid tumors have suggested the efficacy of preventive lamivudine treatment. However, these findings were based on the results from patients with different tumor types and chemotherapy regimens10, 11). We therefore sought to evaluate the frequency and risk factors for HBV reactivation, and to assess the necessity of prophylactic lamivudine, in breast cancer patients receiving adjuvant anthracycline based chemotherapy. This is the first study of the frequency of hepatitis and its outcome, as well as its impact on planned chemotherapy, in HBsAg seropositive breast cancer patients, all of whom were receiving adjuvant anthracycline -containing adjuvant chemotherapy.
In our previous study, we evaluated liver function abnormalities during adjuvant AC chemotherapy in 178 patients with breast cancer. Most of them (97%) were HBsAg negative. We found that 62 (35%) had abnormal liver function. However, only two patients (1%) developed hepatic dysfunction, severe enough to fit the criteria for the diagnosis of acute hepatitis while on chemotherapy20). In this retrospective evaluation of HBsAg positive patients, we found that 60% of the patients developed hepatic dysfunction, with 33% meeting the criteria for the diagnosis of acute hepatitis. The incidence of hepatitis due to HBV reactivation was as high as 21% of all patients. Among them, more than half had severe icteric hepatitis. This result is consistent with several previous studies that reported a 24~28% incidence of HBV reactivation; although the patients and chemotherapeutic regimens differed11, 21).
Several investigators have proposed prophylactic treatment with lamivudine in patients with solid tumor cancers based on the improved outcomes compared to a historical control group10, 11). In a small study on breast cancer, it was reported that the patients in a prophylactic lamivudine group had a significantly lower incidence of hepatitis (12.9% vs. 59.0%), a lower HBV reactivation (6.5% vs. 31.1%) and reduced disruption of chemotherapy (16.1% vs. 45.9%) compared with a control group22). Although there is no consensus on the optimal duration of prophylactic lamivudine therapy, some have recommended that lamivudine be continued until at least 6 weeks after the end of chemotherapy based on previous reports of withdrawal flareups of hepatic dysfunction12).
Our patients were treated with lamivudine after viral reactivation was detected. However, despite lamivudine treatment, improvement of the liver function was slow and consequently, chemotherapy could not be given as planned in 78% of these patients, which was 16% of all 111 patients. Adjuvant therapy is essential for most patients with breast cancer. Anthracycline-based adjuvant chemotherapy is associated with an 11% reduction in the annual odds of relapse and a 16% reduction in the annual odds of dying23). In addition, it is well known that adequate adjuvant therapy is important for a better relapse free survival24, 25). Although we have not analyzed the clinical outcome for cancer, because of the relatively short period of follow-up, it is likely that incomplete chemotherapy could affect the outcome.
For the 14 patients with hepatitis of undetermined cause, we could not identify the disease etiology in this retrospective study. There are several potential causes such as another virus, drugs or alternative agents. Another possibility is that some HBV reactivation might not have been detected due to the timing of the testing. There were eight patients with their HBV DNA tested after their ALT was ≥grade 3. It is well known that viral replication precedes the biochemical flare-up. Therefore, by the time clinical hepatitis was evident, the HBV DNA might have decreased to undetectable levels6, 26). Serial HBV DNA follow-up has been shown to detect HBV reactivation more frequently than conventional monitoring27). In addition, the method used to measure HBV DNA is important; real-time PCR is a more sensitive method, with a detection limit of 4×102 copies/mL18). If we had tested the viral DNA serially, from the beginning of chemotherapy with real-time PCR, we might have been able to detect more cases of hepatitis associated with viral reactivation.
Finally, we failed to detect any clinical factors to predict HBV reactivation during chemotherapy. Previously, the baseline ALT level, the HBV DNA load, the use of anthracycline-containing regimens, and the use of corticosteroids were reported to be risk factors for HBV reactivation21, 28, 29). The univariate analysis showed that older patients and those with abnormal sonographic findings (fatty liver/cirrhotic change) had a greater tendency for HBV reactivation. However, the multivariate analysis identified no clinical factors that could predict HBV reactivation during chemotherapy. Even the steroid treatment was not found to be a significant factor; this might have been because the patients received steroid medications for only a short time per each cycle as an antiemetic.
Our study has the limitation of being a retrospective study. In addition, we did not regularly perform other pertinent examinations, including serial tests for HBV DNA and liver ultrasonography. In addition, detailed investigations of other medications and alternative agents were not available, and they may have been helpful in revealing the etiology of some of the cases with hepatitis of unknown etiology. Despite these limitations, our study clearly showed that hepatitis from HBV reactivation occurs in a significant proportion of patients with HBsAg, and once hepatitis developed, the planned chemotherapy had to be disrupted in most cases even with lamivudine treatment. Our findings suggest that, to proceed with adjuvant chemotherapy as planned in HBsAg positive patients, prevention of HBV reactivation should be considered rather than initiating the treatment after hepatitis has already developed. However, prospective randomized clinical trials are required to determine whether preventive treatment would be more effective and determine other important factors such as the associated cost..
In conclusion, HBV reactivation occurred in breast cancer patients receiving adjuvant chemotherapy; more than 20% of patients who were inactive HBsAg carriers developed hepatitis due to viral reactivation. Most of the affected patients had severe hepatitis and this resulted in either a delay or early termination of chemotherapy. Considering that 16% of HBsAg positive patients could not finish chemotherapy as scheduled, even when lamivudine was used for treatment after detection of the viral reactivation, the option of prophylactic lamivudine should be considered even in the absence of prospective randomized studies.
Notes
This study was supported partly by a grant of the Korean Health 21 R&D Project, Ministry of Heath & Welfare, Republic of Korea (0412-CR01-0704-0001)