Benzalkonium Chloride Induced Bronchoconstriction in Patients with Stable Bronchial Asthma
Article information
Abstract
Background
Although benzalkonium chloride (BAC)-induced bronchoconstriction occurs in patients with bronchial asthma, BAC-containing nebulizer solutions are still being used in daily practice in Korea. The aim of this study was to evaluate the effects of inhaled aqueous solutions containing BAC.
Methods
Thirty subjects with bronchial asthma and 10 normal controls inhaled up to three 600 µg nebulized doses of BAC using a jet nebulizer. FEV1 (forced expiratory volume at one second) was measured 15 minutes after each dose. Inhalations were repeated every 20 minutes until FEV1 decreased by 15% or more (defined as BAC-induced bronchoconstriction) or the 3 doses were administered.
Results
The percent fall in FEV1 in response to BAC inhalation was significantly higher in asthmatics than in normal subjects (p<0.05). BAC administration in subjects with asthma reached a plateau (maximal effect). BAC-induced bronchoconstriction was found in 6 asthmatics (20%), with two responders after the 2nd inhalation and after the 3rd inhalation. The percent fall in FEV1 in response to the 1st inhalation of BAC was significantly higher in asthmatics with higher bronchial hyperresponsiveness (BHR) than in those with lower BHR.
Conclusions
This study suggests that the available multi-dose nebulized solution is generally safe. However, significant bronchoconstriction can occur at a relatively low BAC dose in asthmatics with severe airway responsiveness.
INTRODUCTION
Benzalkonium chloride (BAC) is one of several quaternary ammonium compounds used in pharmaceuticals as antiseptics and disinfectants1). It is also the most common preservative in nebulizer solutions. BAC has been associated with unintended bronchoconstriction after use of aerosolized asthma medications and after occupational exposure1, 2). For example, isotonic ipratropium bromide inhalation solution containing 0.25 mg/mL BAC caused significant bronchoconstriction in patients with asthma (20% drop of FEV1 in 6 of 22 subjects)3), and BAC-induced bronchoconstriction has been documented in successive studies4-6). BAC in nebulizer solutions may also lead to respiratory arrest7).
These reports provoked a worldwide call for the withdrawal of BAC from nebulizer solutions8). Although most BAC-containing nebulizer solutions disappeared from clinical use, 0.5% albuterol (salbutamol) non-sterile solution, which contains 50 µg of BAC per 2.5 mg of albuterol, is still frequently prescribed in everyday practice for the treatment of bronchial asthma in Korea.
We therefore evaluated the effect of BAC inhalation in patients with stable bronchial asthma to assess the safety of BAC-containing nebulizer solutions.
MATERIALS AND METHODS
Subjects
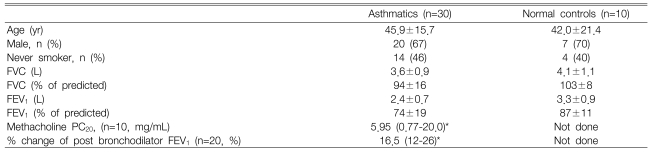
Among patients with stable bronchial asthma who had visited our outpatient clinic, 30 patients with a baseline FEV1 of 65% or greater of the predicted value were selected for this study. Bronchial asthma was diagnosed by the presence of symptoms compatible of bronchial asthma and positive results in a methacholine bronchial provocation test (MBPT) or bronchodilator response (BDR). MBPT was considered positive when the methacholine concentration needed to decrease post-provocation FEV1 by more than 20% of the baseline value (methacholine PC20 FEV1) was less than 25 mg/mL, and BDR was deemed positive when the post-bronchodilator increase of FEV1 was more than 12% of the pre-bronchodilator value. Stable bronchial asthma was defined as asthma with no asthmatic attack resulting in a hospital visit during two recent, consecutive months; no change of medication due to exacerbation; and FEV1 changes of less than 10% of the patient's best FEV1.
Subjects with a history of life-threatening asthma or anaphylaxis were excluded from the study. Other reasons for exclusion were an emergency department visit, hospitalization for asthma within the previous 3 months, a reported use of oral corticosteroids within the previous 3 months, or a respiratory tract infection during or within 6 weeks before the study. Before the study began, subjects had abstained from short-acting β-adrenergic bronchodilators for at least 6 hours, long-acting β-adrenergic bronchodilators for a minimum of 48 hours, short-acting antihistamines for 4 days, and leukotriene modifiers for 48 hours. The control group consisted of 10 adult subjects with normal spirometry and negative MBPT.
This study was approved by the Institutional Review Board of Eulji Hospital.
Study design
Before the provocation study, spirometry was performed to ensure that FEV1 was 65% or greater of the predicted value and within ±10% of the value measured in the previous study. The bronchial provocation test was performed with BAC following a method modified from that used by Asmus and colleagues4). The subject inhaled 3 mL of 0.9% NaCl solution and then 3 mL of 0.9% NaCl solution containing 600 µg of BAC (Sigma, St. Louis, MO, U.S.A.). The solutions were prepared using aseptic techniques and stored at 2 C in an Eppendorf tube. They were allowed to warm to room temperature immediately prior to use. Each 3 mL dose was inhaled using normal tidal breathing through a DeVilbiss 646 nebulizer (DeVilbiss Co., Somerset, PA, U.S.A.). Vmax22® spirometry (SensorMedics, Yorba Linda, CA, U.S.A.) was performed 15 minutes after the inhalation of each dose began. The provocation was repeated every 20 minutes until FEV1 decreased by at least 15% or until a maximum of three doses had been administered. BAC-induced bronchoconstriction was defined as a decrease of 15% or more in FEV1 after each BAC inhalation. To compare differences in BAC-induced bronchoconstriction according to airway responsiveness, asthmatics with PC20 less than 4.0 mg/mL were also analyzed as a separate subgroup.
The subjects were given 100 µg of albuterol (Ventolin® evohaler, GlaxoSmithKlein, Middlesex, U.K.) with a metereddose inhaler to reverse bronchoconstriction if it occurred or on request. The albuterol dose was repeated, if necessary, 20 minutes later.
Statistics
Basal characteristics between the asthmatic and control groups were compared by a Student's t-test for continuous variables and a chi-square test for categorical variables. BACinduced decreases in FEV1 and differences in bronchoconstriction between groups were divided by means of airway sensitivity and compared using a paired t-test and a Mann-Whitney test, respectively. We used the SPSS software package (SPSS 11.0.0, SPSS Inc., Chicago, IL, U.S.A.) and a p value of <0.05 was considered statistically significant.
RESULTS
There were no significant differences in age, sex, FEV1 % predicted value, or smoking status between asthmatic patients and control subjects (Table 1). A total of three BAC inhalations, for a cumulative dose of 1,800 g, was given to 30 patients and 10 normal control participants; among the 30 patients, two did not inhale the 3rd BAC solution because their decreased FEV1 exceeded 15% of the baseline value.
The mean FEV1 values at baseline, 1st, 2nd and 3rd inhalation were 2.36 L±0.74 L, 2.26 L±0.64 L, 2.18 L±0.63 L and 2.17 L±0.65 L in asthmatics, and 3.32 L±0.91 L, 3.33 L±0.82 L, 3.33 L±0.90 L and 3.34 L±0.85 L in the control group, respectively. In asthmatics, the mean percent fall in FEV1 after each inhalation was 2.69%, 5.36%, and 5.30%, respectively. FEV1 after the 1st and the 2nd inhalations decreased significantly from the previous value (p=0.001, p=0.001), but there was no difference between FEV1 after the 2nd and 3rd inhalation (p=0.973) (Figure 1). There were no significant changes in FEV1 in the control group.
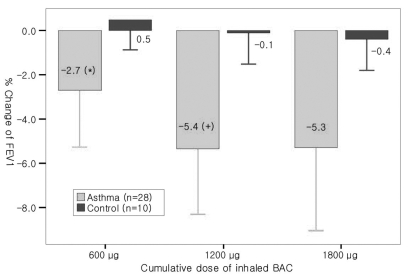
Course of mean % change in FEV1 after inhaling BAC in asthmatics and controls. Changes of FEV1 after BAC inhalation were cumulative up to 1200 µg and reached a plateau. There was no significant bronchoconstriction in the control group.
*: p<0.01, compared with baseline value.
†: p<0.01, compared with 600 µg BAC
BAC-induced bronchoconstriction (a decrease of FEV1 by 15% or more) was found in 6 patients (6/30, 20%), with 2 responders after the 2nd inhalation and 4 after the 3rd inhalation. Patients with BAC-induced bronchoconstriction complained of coughing, chest discomfort, or dyspnea shortly after BAC inhalation, and these symptoms were relieved effectively by short-acting β2-agonist inhalation.
BAC-induced FEV1 changes in the subgroup with high airway sensitivity (PC20 less than 4.0 mg/mL) were only significantly different from other asthmatics after the 1st BAC inhalation (p=0.038) (Figure 2).
DISCUSSION
BAC-induced decreases in FEV1 were cumulative up to 1,200 µg and the reached a plateau, with significant bronchoconstriction (decrease of FEV1 ≥15%) occuring in 6 of the 30 patients with stable bronchial asthma (6/30, 20%). BAC-induced bronchoconstriction was initially more severe in patients with more sensitive airway responsiveness, but this trend disappeared with additional BAC doses. BAC-induced bronchoconstriction was easily reversed with a short-acting β2-agonist.
BAC-induced bronchoconstriction is cumulative, prolonged, and determined by basal airway responsiveness5, 6). Zhang and colleagues reported that the range of BAC PC20 FEV1 was 0.03 to 5.5 µmol (1 µmol is equivalent to 354 µg of BAC). When they repeatedly doubled the BAC concentration from 0.044 to 5.64 µmol, bronchoconstrictions (≥10% fall in FEV1) developed in 25 of 28 subjects during a BAC inhalation challenge, and 17 of 28 subjects showed at least a 20% decrease of FEV1. The dose-response to BAC was steep and did not appear to plateau. There was also a significant correlation between histamine PC20 FEV1 and BAC PC20 FEV16).
The results of Asmus and colleagues confirmed the observations of previous investigators; 10 (55%) of 18 subjects showed at least a 20% decrease in FEV1 4). They increased the inhaled dose of BAC from 600 to 2,400 µg with respective inhalations of 600 µg of BAC at 20-minute intervals. This cumulative manner of BAC inhalation more closely approximates the clinical situation than the method employed in the study of Zhang and colleagues. In the latter study, the authors intended to calculate BAC PC20 FEV1, and the dose of BAC was doubled as in the dosing method of the methacholine provocation test.
There are some notable differences between the results of our study and previous results. First, we found that bronchoconstriction due to BAC inhalation reached a plateau after a cumulative BAC dose of 1,200 µg. Whereas asthmatics with moderate to severe bronchial hyperresponsiveness were selected6), our study included patients with mild bronchial hyperresponsiveness, who may be less sensitive to BAC inhalation, allowing dosing to reach a plateau. Second, the difference in BAC-induced bronchoconstriction was not maintained with additional doses in patients more severe airway responsiveness (PC20 less than 4.0 mg/mL). A significant difference was observed only at the 1st inhalation of BAC 600 µg, with bronchoconstriction reaching a plateau earlier in these patients (Figure 2). Further studies are needed to clarify this difference.
The mechanism of BAC-induced bronchoconstriction is not clear1). The main controversy is whether the dominant mode of action is IgE-dependent or non-IgE-dependent mediator release. A positive intradermal test result with BAC implies an IgE-mediated response9, 10), but BAC can elicit non-IgE mediated histamine release from rat mast cells and its bronchoconstriction can be blocked by antihistamines9, 11).
The bronchoconstrictive effects of BAC were originally described after inhalation of an ipratropium bromide nebulizer solution that contained 250 µg/mL BAC3). Ipratropium is now only available as a preservative-free solution. Previously, a non-sterile, screwcap unit-dose albuterol nebulizer solution that contained 300 µg of BAC per 2.5 mg dose of albuterol was on the market, but it is no longer available.
Metered dose inhalers now contain no preservatives. A recently introduced propellant-free and multi-dose inhalation device, the Respimat® Soft Mist Inhaler™ (SMI), uses BAC and EDTA as preservatives. The amount of BAC delivered to the lungs in a single actuation is 0.44 µg, which is approximately 200 times lower than that delivered by wet nebulizer solutions. Patel et al12) reported that the decreases of FEV1 in asthmatics with airway hyper-reactivity by four actuations of an aqueous placebo that contained no bronchodilator (12 µL water + 5.5 µg EDTA + 1.1 µg BAC/actuation) via Respimat® SMI were not different from decreases of FEV1 by normal saline (-0.121 L vs. -0.094 L; 90% CI -0.107~0.052 L, within a pre-determined equivalence region of ±0.15 L).
In clinical practice in Korea, rapid-acting β2-agonists for nebulization are available in sterile unit-dose screwcap vials and a nonsterile multidose dropper bottle (Ventolin® respiratory solution, GlaxoSmithKline, U.K.). The latter contains 50 µg BAC in each 2.5 mg dose of albuterol, and its manufacturer recommends using 2.5-5 mg of albuterol by nebulization in a proper clinical setting. According to GINA (Global INitiative for Asthma management and prevention) guidelines for hospital-based management of asthma exacerbation, rapid acting β2-agonist, generally administered by nebulization, can be given at one dose every 20 minutes for 1 hour13). Therefore, if a nonsterile multidose dropper bottle is used, inhalation of BAC 150-300 µg is possible over 1 hour.
In the present study, the median value of the % decrease of FEV1 after inhalation of 600 µg BAC was 2.86% (range: -5.0~11.9). Thus, the currently available multi-dose dropper bottled albuterol is generally safe if used according to the recommended directions. However, for asthmatics with severe airway responsiveness, a paradoxical bronchoconstriction can occur at a relatively low BAC dose. In a clinical situation, if there is no response to a sufficient dose of BAC-containing bronchodilator solution or if dyspnea is paradoxically aggravated, BAC-induced bronchoconstriction should be considered. A bronchial provocation test with the actual dose of BAC included in currently-used nebulizer solutions to patients with more severe bronchial hyperresponsiveness will show more clinically meaningful results.
Notes
This study was supported by a grant from the Korea Health 21 R&D Project by Ministry of Health & Welfare. R.O.K. (03-PJ10-PG13-GD01-0002)