Differences in Insulin Sensitivity and Secretory Capacity Based on OGTT in Subjects with Impaired Glucose Regulation
Article information
Abstract
Background
This study examined whether defects in insulin secretion contribute to the development and progression of type 2 diabetes mellitus (T2DM).
Methods
Plasma insulin and glucose were measured after a glucose tolerance test to calculate the insulinogenic index (IGI) and the HOMA-IR Homeostasis model assessment of insulin resistance in subjects with normal glucose tolerance (NGT), prediabetes (preDM, n=49), and T2DM patients with disease duration <1 year (n=84), 1~5 years (n=45), or >5 years (n=37). Plasma proinsulin and adiponectin levels were also measured as a parameter of insulin secretion and resistance.
Results
The mean HOMA-IR increased and the adiponectin levels decreased relative to the deterioration of glucose tolerance in NGT and preDM subjects. However, differences in the HOMA-IR were not related to disease duration in T2DM subjects. The mean IGI was similar in NGT and preDM subjects, but there were significant deteriorations in IGI relative to the duration of diabetes.
Conclusions
Defects in both insulin sensitivity and insulin secretion contribute to T2DM, but decreased insulin secretion may be more important in the development and progression of T2DM.
INTRODUCTION
Several metabolic problems are associated with the development of type 2 diabetes (T2DM). In particular, insulin resistance and decreased insulin secretion contribute to the pathogenesis of T2DM1). Insulin resistance is a decrease in the endogenous sensitivity to insulin action. Endogenous insulin secretion suppresses hepatic glucose biosynthesis, increases glucose uptake and glycogen synthesis in target cells, and reduces the lipolysis of adipocytes2, 3). However, increased insulin resistance impedes normal insulin activity, leading to hyperinsulinemia and, eventually, T2DM4-6).
Deteriorating insulin secretion results from β cell dysfunction due to glucotoxicity, lipotoxicity, βcell exhaustion, or impaired proinsulin biosynthesis7). Deteriorating insulin secretion is seen in all T2DM patients, particularly in the early phase8, 9).
In fact, decreased insulin secretion and increased insulin resistance precede the development of clinical hyperglycemia10). Thus, early T2DM shows increases in endogenous insulin levels with increasing insulin resistance, maintaining normal glucose tolerance. However, progressive reductions in insulin secretion eventually cannot compensate for insulin resistance, and progresses to T2DM11).
Deteriorating insulin secretion may be actually be required for T2DM development, since, at least theoretically, increased insulin production could always compensate for increases in insulin resistance. Therefore, here we measured indices for insulin resistance and insulin secretion in Korean prediabetic and T2DM subjects using an oral glucose tolerance test, and then compared these indices according to the severity of glucose tolerance and disease duration.
MATERIALS AND METHODS
Study Subjects
This study was performed from May 2002 to September 2003, inclusive, on 251 subjects with suspected or established T2DM at the Department of Endocrinology and Metabolism, Kyung Hee University Hospital. A physical examination and oral glucose tolerance test (OGTT) were performed on all subjects. Patients with a fasting plasma glucose ≥11.1 mmol/L (200 mg/dL) were excluded from the study population to rule out insulin secretory dysfunction due to severe glucotoxicity.
Methods
In all subjects, the height, weight, body mass index, waist circumference, resting blood pressure, etc. were measured. The OGTT was performed after a minimum of 8 hours fasting, and fasting plasma glucose (Glc0), insulin (Ins0), C-peptide (Cpep0) and plasma proinsulin and adiponectin concentrations were measured to gauge β cell function and insulin resistance. After administering 75 g glucose orally, the plasma glucose concentration was measured at 30, 60, 90, and 120 minutes (Glc30, Glc60, Glc90, Glc120), and plasma insulin (Ins30) and C-peptide (Cpep30) concentrations were measured 30 minutes after glucose administration. Plasma glucose levels were measured using a Biosen analyzer (Biosen 5030; EKF-diagnostic GmbH, Magdeburg, Germany) and plasma insulin (Daiichi RI Co., Tokyo, Japan), C-peptide (Daiichi RI Co.), adiponectin (Linco Research, MO, USA), and proinsulin (Linco Research) were measured using commercially available radioimmunoassay kits.
Subjects were divided into three groups according to OGTT results: normal glucose tolerance (NGT), prediabetes (preDM), which included impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT), and type 2 diabetes mellitus (T2DM). The diagnosis of T2DM was made according to the diagnostic criteria of the American Diabetes Association12). We also calculated insulin resistance using the OGTT, HOMA-IR Homeostasis model assessment of insulin resistance and insulin secretion with the Insulinogenic Index (IGI) as follows13).
HOMA-IR = (Ins0 × Glc0)/22.5
IGI = (Ins30 - Ins0) / 18(Glc30 - Glc0)
Ins0 : fasting plasma insulin (mIU/L)
Ins30 : insulin 30 min after glucose intake (mIU/L)
Glc0 : fasting plasma glucose (mmol/L)
Glc30 : plasma glucose 30 min after glucose intake (mmol/L)
Patients diagnosed with T2DM were classified according to disease duration; less than 1 year (DM <1 yr), 1~5 years (DM 1~5 yr), or over 5 years (DM >5yr). Therefore, we had a total of 5 subgroups, NGT, preDM, DM <1 yr, DM 1~5 yr, and DM >5 yr for comparing insulin resistance and insulin secretion. For subjects taking oral hypoglycemic agents or insulin, a 2-day drug rest period was allowed prior to the OGTT to minimize the effect of the drugs.
Statistical Analysis
The Statistical Package for Social Science (SPSS, Version 13.0; SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The Student's t test, one way ANOVA, ANCOVA, and chi-square tests were used.
RESULTS
Clinical Characteristics
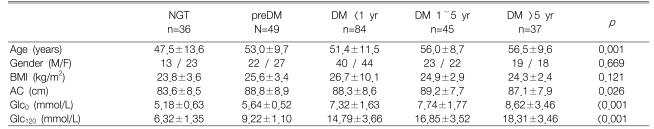
Of the 251 subjects, 36, 49, and 166 were diagnosed as NGT, preDM, and T2DM, respectively. There were 84, 45, and 37 patients with DM <1 yr, 1~5 yrs, and >5 years, respectively (Table 1).
There was no significant difference between the subgroups according to gender, but age and waist circumference showed an increasing trend with lower glucose tolerance. However, waist circumference showed this trend because the waist circumference of the NGT group was extremely low. The post hoc test and ANOVA excluding the NGT group showed no statistically significant differences between the groups (p=0.775).
Subgroup analysis of insulin resistance and insulin secretion
In each group, the IGI and plasma adiponectin concentration decreased significantly with increasing glucose tolerance, and the HOMA-IR and plasma proinsulin concentration increased significantly with increasing glucose tolerance (Table 2). There was no change in statistical significance after adjusting for specific co-variants that might mediate an effect on insulin resistance or insulin secretion. However, indices of insulin resistance and insulin secretion did not show a linear relationship with the exacerbation of glucose tolerance or increased disease duration. For example, IGI was similar in the NGT and the preDM groups, and decreased with increasing T2DM disease duration (Figure 1). Although HOMA-IR increased with increasing glucose tolerance, the trend was only significant after adjusting for age and abdominal circumference by ANCOVA. Adiponectin, but not proinsulin, showed a significant change after adjusting for covariances in the NGT and the preDM groups (p=0.006). Neither proinsulin and adiponectin levels were associated with T2DM disease duration (data not shown).

Crude and adjusted geometric means of differences in insulin secretion and insulin resistance indices

Differences in insulin secretion capacity (IGI) and the insulin resistance index (HOMA-IR) according to glucose tolerance or T2DM duration. Changes in IGI were not significant in NGT and preDM subjects but decreased progressively with disease duration, whereas HOMA-IR values progressively increased with glucose tolerance aggravation, but did not change with disease duration.
DISCUSSION
Increased insulin resistance and deterioration of insulin secretion contribute to T2DM, but their interaction and role in disease progression is unclear14, 15). Although certain pathophysiology and clinical features in T2DM are similar regardless of ethnicity, Koreans more frequently have non-obese T2DM than Caucasians, and the absolute number of β cells is lower, meaning that insulin deficiency usually plays a more important role than insulin resistance16, 17). These differences suggest that specific treatment guideline for Korean patients may be required, especially since appropriate treatment may dramatically influence the clinical course of the disease.
This study examined a large number of patients, as well as multiple indices (adiponectin, proinsulin, the HOMA-IR and IGI) in different Korean prediabetic and T2DM patients to compare differences in insulin resistance and insulin secretion. Fasting plasma adiponectin levels are relatively independent of changes in insulin secretion, but decreased with increasing insulin resistance18-20). In addition, β cell dysfunction leads to decreased cleavage of the insulin precursor, which could be assessed by increased levels of proinsulin and its derivatives21).
Our results showed that IGI decreased and proinsulin levels increased with increasing glucose intolerance, indicating the presence of deficient insulin secretion. IGI was noticeably lower in T2DM than the NGT and preDM groups, and decreased continuously with T2DM duration. In contrast HOMA-IR increased and adiponectin levels decreased with increasing glucose tolerance, indicating insulin resistance. Although there was a significant difference in HOMA-IR and adiponectin between the NGT group and the preDM groups, there was no further significant change after T2DM manifestation. Thus, insulin secretion from β cells seems relative well preserved in preDMs. However, T2DM patients did not show further increases in insulin resistance, but did show decreased insulin secretion. These results suggest that decreased insulin secretion is more significant than increases in insulin resistance in Koreans.
In obese subjects with NGT, decreased β cell function and insulin secretion were not associated with insulin resistance or decreased insulin sensitivity22). Thus, increased insulin resistance and decreased insulin secretion do not always progress simultaneously, which might explain the trends in IGI and HOMA-IR observed in this study.
One limitation of our study is that changes in insulin resistance and insulin secretion was not compared over time in the same subject. Our subgroups also were relatively small, and the preDM group was not further subdivided. Although HOMA-IR and IGI are reasonable measures of insulin resistance and insulin secretion, the insulin clamp and IVGTT are more accurate. In general, adiponectin, proinsulin, and other laboratory markers were consistent with the IGI and HOMA-IR results, although with some variability.
Despite these limitations, our data still illuminate the pathophysiology of insulin resistance and secretion in Korean prediabetics and type 2 diabetes patients. In particular, the decrease in insulin secretion seems more important in the progression and exacerbation of diabetes than insulin resistance. However, a future prospective study to overcome the limitations of this study, as well as a molecular biological study, will be needed to further clarify the changes in insulin resistance and secretion in the pathophysiology of early phase T2DM.
Notes
This study was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant No. A050463).