Primary Retroperitoneal Mucinous Cystadenocarcinoma: A Case Report and Review of the Literature
Article information
Abstract
Primary retroperitoneal mucinous cystadenocarcinoma is a rare tumor. Only about 30 such cases have been reported in the worldwide literature, and a few Korean cases have been reported. The pathogenesis is not clear, and coelomic metaplasia of the retroperitoneal mesothelium has gained wide support. There is no consensus on the appropriate treatment, but surgical exploration is needed for the diagnosis and treatment, and adjuvant chemotherapy may be recommended following complete surgical excision. The long-term prognosis has not been established.
We report here on a 32-year-old woman who was diagnosed as having a retroperitoneal mucinous cystadenocarcinoma with mural nodules of sarcomatoid change. Tumor excision and adjuvant chemotherapy were done and the patient is doing well without any evidence of recurrence at 42 months postoperatively.
INTRODUCTION
Primary retroperitoneal mucinous cystadenocarcinoma is a rare tumor with only about 30 reported cases in the English literature. The histogenesis of this unusual neoplasm is not clear. Surgical exploration is needed for the diagnosis and treatment, and many authors recommend extensive excision including total hysterectomy and bilateral salpingo-oophorectomy with enucleation of retroperitoneal tumor1-9). Adjuvant chemotherapy is sometimes administered following complete surgical excision4, 6, 7, 10, 11). However, the most desirable treatment for this rare tumor is still controversial. We report here on a patient who had tumor excision and adjuvant chemotherapy, and the patient is without evidence of recurrence 42 months after surgery. We also review the previous literature that's concerned with this neoplasm.
CASE REPORT
A 32-year-old married woman was referred to the surgical unit because of a self-palpable mass in her left lower abdomen for over 1 month and the abdominal pain for the recent several days. Physical examination revealed a slightly tender, ill-defined mass about 10 cm in size over the left lower abdomen. The laboratory tests, including the complete blood count, the chemistry profile, urinalysis and chest X-ray, were all within normal limits. Abdominopelvic computed tomography scanning revealed a huge unilocular cystic mass with an enhancing solid portion, and this was probably located in the retroperitoneal space (Figure 1A, 1B). There was no evidence of extracystic extensi on or distant metastasis.
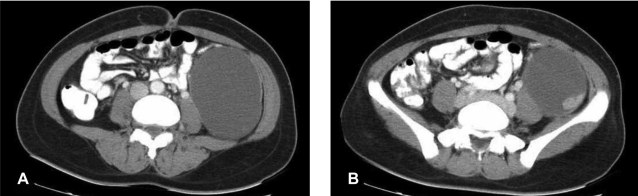
(A) Abdominopelvic computed tomography shows a large unilocular cystic mass in the left retroperitoneal space. (B) There is an enhancing solid portion in the posterolateral aspect of the inner surface of the cyst.
Laparotomy revealed a grossly normal uterus, 2 ovaries, 2 fallopian tubes and an appendix. There were no abnormal findings in the liver, stomach, small bowel, large bowel and spleen. A large, encapsulated cystic mass about 10 cm in diameter was found in the retroperitoneum. It was no connection to the bowels and other organs. The descending colon was displaced medially to the mass, and no ascites was found. Excision of the cystic mass was done. The capsule did not rupture during operation. Hysterectomy, salpingooophorectomy, appendectomy and lymphadenectomy were not performed.
The gross pathologic findings showed a 15×8×7 cm sized, 606 gram cystic mass. It was unilocular and contained dark brown viscous material. The inner surface of the cyst was smooth except for three solid mural nodules that were 0.8, 1 and 1.5 cm in diameter, respectively. The cystic wall measured 0.7 cm at the maximum thickness. There was no evidence of capsular rupture. The mural nodules were soft and brownish pink. Microscopically, the cyst was lined by atypical columnar cells of mucinous cystadenocarcinoma (Figure 2A, 2B). The solid mural nodules showed highly pleomorphic spindle cells and anaplastic giant cells with numerous atypical mitosis, and there were intervening atypical glands of adenocarcinoma (Figure 3A). The spindle and giant cells were positive for cytokeratin, vimentin and smooth muscle actin, but negative for S-100 protein and desmin (Figure 3B, 3C). The carcinoma cells were positive for cytokeratin and they were negative for vimentin, smooth muscle actin, S-100 protein and desmin. The diagnosis of the mass was primary retroperitoneal mucinous cystadenocarcinoma with mural nodules of sarcomatoid change.
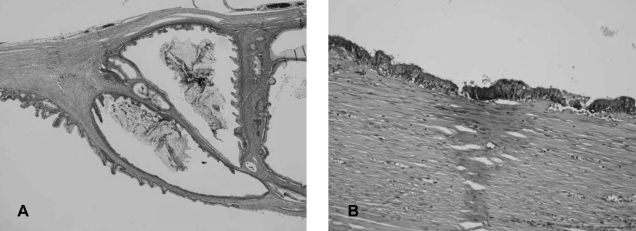
(A) The inner wall of the cyst was lined by atypical columnar cells of mucinous cystadenocarcinoma (H&E, ×100). (B) A positive result for mucicarmin staining in the adenocarcinoma cells (×200).
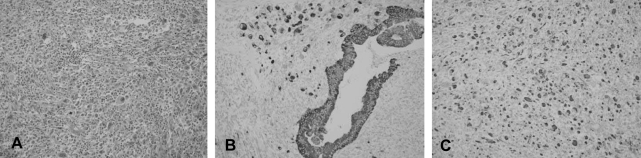
(A) The solid mural nodules showed highly pleomorphic spindle cells and anaplastic giant cells with numerous atypical mitosis (H&E, ×200). (B) The adenocarcinoma cells, spindle cells and giant cells were positive for cytokeratin (×200). (C) The spindle and giant cells were positive for vimentin (×200).
The serum tumor markers were not checked before tumor excision. At 10 days after operation, the serum concentration of CA125 was elevated (76.95 IU/mL), which decreased to within normal limits 3 months later, and the serum concentration of CEA was normal.
The patient had an uneventful postoperative recovery. Adjuvant chemotherapy with six courses of cyclophosphamide 600 mg/m2 and cisplatin 60 mg/m2 was administered to the patient. There was no evidence of recurrence on the follow-up abdominopelvic computed tomography 42 months after the operation. Also, the CA125 level was maintained at a normal level.
DISCUSSION
Primary retroperitoneal mucinous cystadenocarcinoma is a rare tumor. The first case was described in 1977 by Roth et al.12), and about 30 cases have been reported in the English medical literature to date.
Several hypotheses have been suggested to explain the histogenesis of primary retroperitoneal mucinous cystic neoplasm, and they are heterotopic or supernumerary ovary11, 12), retroperitoneal teratoma13), intestinal duplication and coelomic metaplasia. To date, the hypothesis that has gained increasing support is coelomic metaplasia, that is, retroperitoneal mucinous cystadenocarcinomas arise from invaginations of the peritoneal mesothelium, with subsequent mucinous metaplasia1, 3, 4, 5, 12, 14-16). The ultrastructural findings and immunohistochemical observations support this hypothesis3, 4).
The age at diagnosis ranges from 17 to 86 years and only 2 of the reported cases were male15). The patients usually visit the hospital with a complaint of abdominal pain or a palpable mass. Radiologic tests including ultrasonography, computed tomography or magnetic resonance imaging are used to localize the tumor and evaluate its nature. The papillary nodules demonstrated within a cyst by ultrasonography or computed tomography have been reported to suggest malignancy16). However, it is often difficult to differentiate a benign from a malignant neoplasm, or even to determine the origin site of the tumor with using the preoperative radiologic images7). Serum tumor markers rarely assist in the diagnosis or follow-up7). Motoyama et al. suggested that the presence of glandular epithelial cells and high levels of CEA in the cystic fluid was very useful for making the diagnosis15). Laparotomy, tumor excision and pathologic examination are inevitable to make a correct diagnosis.
The treatment of primary retroperitoneal mucinous cystadenocarcinoma remains controversial. The immunohistochemical findings of this neoplasm resemble those of its ovarian counterpart; thus, a large number of authors recommend management according to the treatment protocol for ovarian neoplasms, including the staging procedure3, 7). The tumor was removed surgically in nearly all the previously reported cases. Although almost all the cases, except the one reported by Uematsu16) were diagnosed in the early stage, there have been many relapses cases in the literature6, 10-14). Roth et al. reported on a patient who had undergone only tumor excision and he died of spreading disease 6 months postoperatively12). Two patients received tumor excision and adjuvant chemotherapy, but one patient had a paraovarian recurrence 21 months after surgery10) and another patient died of disease spread 4 months after surgery11). The patient with histologically borderline malignancy and who was reported on by Banerjee underwent tumor excision, left salpingo-oophorectomy and descending colon resection, and mediastinal metastasis developed 4 years later14). Mikami et al. noted a patient who experienced rupture of the tumor capsule during the operation with subsequent peritoneal tumor implantation, and the patient died 18 months after the initial surgery6). For the Korean case reported on by Song et al., the tumor was removed laparoscopically after cyst aspiration. Mesenteric lymphadenopathy was found 10 months after surgery and the patient eventually expired13). Five of the reported recurrent cases didn't undergo extensive surgical excision, including hysterectomy and bilateral salpingo-oophorectomy 10-14). Hysterectomy and bilateral salpingo-oophorectomy were conducted after tumor excision in about half of the reported cases1-9), and these case were alive without evidence of recurrence 3 to 36 months postoperatively, except for one case6). So, extensive exploratory laparotomy, including removal of both ovaries and the uterus with extirpation of the retroperitoneal tumor, may improve the prognosis and prevent recurrence1, 2, 5, 11, 17).
Some authors take an opposite view18, 19). The resected genital organs in several reports showed no evidence of tumor involvement or infiltration1-5, 7-9). The only three of all the reported cases showed long-term survival (>5 years) of 6 years, 5 years and 5 years, respectively, and these cases had not undergone hysterectomy and bilateral salpingo-oophorectomy16, 18, 19). Law et al. recommended only tumor excision for young women who have no evidence of disease dissemination and who hope to stay fertile19).
Six cases, including the present case, have reported tumor that had one or more mural nodules6, 8, 9, 11, 12), and these nodules satisfied the diagnostic criteria presented by Baergen. These mural nodules were composed of poorly differentiated adenocarcinoma, sarcomatoid or sarcoma. Mikami et al. reported a patient with primary retroperitoneal mucinous cystadenocarcinoma with a mural nodule, and this nodule was composed of pleomorphic sarcomatoid cells with mitotic figures6). The patient died of her disease at 18 months postoperatively. The authors commented that intraoperative rupture of the cyst in this case may have resulted in its peritoneal dissemination and recurrence. Two patients with mural nodules died of disease at 4 months and 6 months postoperatively, respectively11, 12). One case showed no evidence of recurrence 18 months postoperatively9). Cases with a mural nodule may have an aggressive prognosis like ovarian cancers with a mural nodule; therefore, all mural nodules warrant careful histological assessment and careful postoperative follow-up6, 8). Although our patient was diagnosed as having a neoplasm with mural nodules of sarcomatoid change, she is doing well without evidence of recurrence 42 months postoperatively.
Cystic tumor rupture during operation may occur, and malignant cells may exist in the spilled fluid; this results in peritoneal dissemination. Among the patients with cystic rupture4, 6), one patient died 18 months postoperatively6). In contrast, another patient reported by Tenti et al. was free of tumor 33 months postoperatively, and that patients underwent adjuvant chemotherapy4). In the literatures, adjuvant chemotherapy was performed in 5 patients after tumor resection4, 6, 7, 10, 11). One of them underwent cytoxan chemotherapy for 21 months, but paraovarian recurrence developed10). 2 other patients died 4 months and 18 months after surgery, respectively, of widespread metastasis6, 11). The advantage of adjuvant chemotherapy has not been proved, and tumor might be hard to cure when it recurs, but patients may have benefit from chemotherapy if the cyst ruptures or if extracystic extension is present16).
In the present case, tumor resection and adjuvant chemotherapy were performed. Capsular rupture did not occur, but 3 mural nodules of sarcomatoid change were observed. The patient has no evidence of recurrence on follow-up computed tomography and according to the level of tumor marker. We recommend careful histologic examination of the excised specimen and if possible, aggressive management with extensive surgical excision and adjuvant chemotherapy to prevent recurrence, and consideration must be given to the patient's age, the extent of disease and the histologic findings. Primary retroperitoneal mucinous cystadenocarcinoma is a rare tumor and in all reported cases, the duration of follow-up was not more than 5 years. Long-term follow-up is essential to evaluate the course and prognosis of this disease.