Ischemia-modified albumin (IMA) is not useful for detecting myocardial ischemia during symptom-limited exercise stress tests
Article information
Abstract
Background/Aims
We examined the ischemia-modified albumin (IMA) level during exercise in patients with coronary artery disease (CAD).
Methods
Forty patients with a history of chest pain underwent both symptom-limited treadmill exercise stress testing and coronary angiography within one week. During the treadmill tests, blood samples were obtained at baseline and 5 min after exercise to measure the serum IMA level.
Results
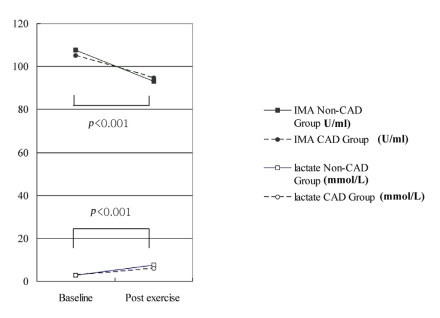
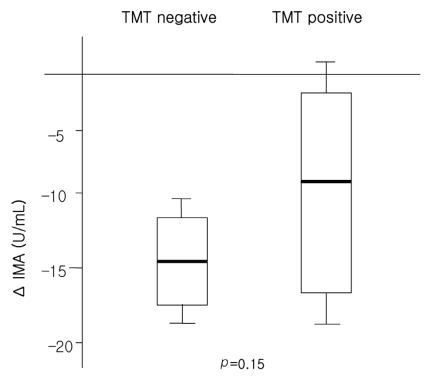
Of the 40 patients, fourteen (35%, CAD group) had significant coronary artery stenosis, while the other 26 (65%, non-CAD group) did not. The baseline and post-exercise IMA levels in the two groups did not differ significantly (105.2±7.2 vs. 107.7±6.7 U/mL at baseline and 93.1±10.1 vs. 94.8±5.7 U/mL at post-exercise in the CAD and non-CAD groups, p=0.29 and 0.57, respectively). The changes in IMA after exercise did not differ either (-10.4±7.5 vs. -14.0±7.6 U/mL in the CAD and non-CAD groups, respectively, p=0.10). Similarly, the change in IMA between the exercise ECG test positive (TMT positive, n=9) and negative (TMT negative, n=20) groups did not differ (-14.63±5.19, vs -8.50±9.01 U/mL, p=0.15, in the TMT positive and negative groups, respectively).
Conclusions
Our results suggest that IMA has limitation in detecting myocardial ischemia during symptom-limited exercise stress tests.
INTRODUCTION
Ischemia-modified albumin (IMA) has emerged as a useful diagnostic modality for evaluating chest pain, especially in patients with acute coronary syndrome1). During an ischemic attack, a lack of oxygen in localized areas of the heart induces a series of reactions involving free radicals leading to an N-terminal structural change in albumin, producing IMA2). Recently, several studies reported a high negative predictive value and high sensitivity for detecting patients with acute coronary syndrome presenting to the emergency department with chest pain3-7). Other studies demonstrated that the serum level of IMA increased rapidly after transient balloon occlusion during percutaneous coronary intervention (PCI)8-10). In contrast, some studies have suggested that the change in IMA in response to ischemia in organs other than the heart is not the same as that in ischemic myocardium, such as in skeletal muscle ischemia and bowel ischemia11, 12). In reality, most patients with stable angina complain of effort-related chest pain, not resting pain. Several studies have examined the meaning of serum IMA in exercise-induced myocardial ischemia and reported that IMA was not useful for identifying myocardial ischemia during exercise13, 14); however, most of these studies used non-invasive tests, such as exercise stress tests, to provide evidence of myocardial ischemia. Such non-invasive tests are limited in their ability to address the issue because of false positive or negative results. Thus, in this study, we examined the meaning of the IMA level during exercise with greater precision by performing both exercise ECG testing and coronary angiography.
MATERIALS AND METHODS
The subjects included 40 patients with a history of chest pain. Patients with the following conditions were excluded from the study: (1) leg claudication; (2) a history of myocardial infarction; (3) a recent (within four weeks) history of PCI; (4) a history of stroke; (5) renal insufficiency (serum creatinine ≥ 2 mg/dL); (6) cancer or other active inflammation; and (7) baseline ECG abnormalities that might complicate the interpretation of exercise ECG test results, such as a left bundle branch block, Wolff-Parkinson-White syndrome, and ST depression. All of the patients underwent both symptom-limited treadmill exercise stress testing and coronary angiography within one week. During the treadmill tests, blood samples were obtained at baseline and 5 min after exercise to measure the serum IMA and lactate levels.
Treadmill Exercise Stress Test (TMT)
All of the patients underwent symptom-limited treadmill exercise ECG testing using the Bruce protocol13). The test was terminated if the patient complained of fatigue, marked dyspnea, exercise-limiting angina, dizziness with a documented systolic blood pressure drop of more than 20 mmHg, or marked ST-segment depression (> 3 mm). The exercise ECGs were interpreted by an independent physician. The patients were declared positive for myocardial ischemia if they showed ≥ 1-mm horizontal or down-sloping ST-segment depression at 80 ms after the J-point during the exercise or recovery period. If they showed no significant ST-segment change, the patients were declared negative for myocardial ischemia or placed in the indeterminate group depending on whether their heart rate (HR) reached more than 85% of the estimated maximum value. Blood samples were obtained before exercise (baseline) and 5 min after the cessation of exercise. The patients were assigned to the TMT positive or negative group according to their TMT result.
Coronary Angiography
Coronary angiography was performed within one week of the exercise treadmill test. The femoral or radial artery was used as the puncture site. Quantitative coronary angiography (QCA) was used to determine the degree of stenosis in the major branches of the coronary arteries. If a narrowing lesion with ≥ 50% stenosis was detected by QCA, coronary artery disease (CAD) with significant stenosis was deemed present. Patients were assigned to the CAD or non-CAD group according to whether they had significant CAD on the coronary angiogram.
Analysis of Blood Samples
Blood was collected in Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ, USA). The samples were frozen at -70℃ within 2 h and stored until analysis. Serum IMA was measured using the Albumin Cobalt Binding (ACB) Test (Ischemia Technologies, Denver, CO, USA) using a Synchron LX20 chemical autoanalyzer (Beckman Coulter, Fullerton, CA, USA). The total intra-assay coefficient of variation (CV) was 2.36~3.29% while the inter-assay CV was 3.01~6.35% at 55-132 U/mL for the quality-control materials. The lactic acid concentration was measured using a Nova M7.
Data Analysis
The serum IMA and lactate levels before and after exercise were compared in both the CAD/non-CAD groups and the TMT-positive/-negative groups.
Statistical Analysis
Student's t-test was used to compare continuous variables (expressed as the mean±SD). For discrete variables, χ2 analysis was used. For non-parametric testing, the Mann-Whitney U-test was used to compare continuous variables in two groups. All p-values less than 0.05 were considered statistically significant.
RESULTS
Coronary Angiography
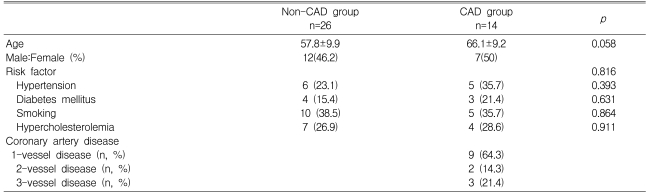
Of the 40 patients, 26 showed no significant narrowing (the non-CAD group) whereas the other fourteen patients showed evidence of significant stenosis in at least one coronary artery (the CAD group). Age, sex, and the proportion of risk factors for CAD did not differ between the two groups. In the CAD group, nine patients (64.3%) had 1-vessel disease, two patients (14.3%) had 2-vessel disease, and three patients (21.4%) had 3-vessel disease (Table 1).
Exercise ECG Testing
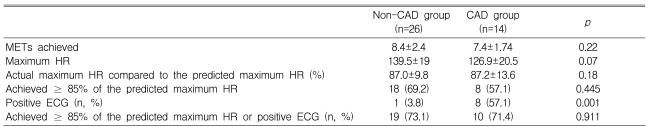
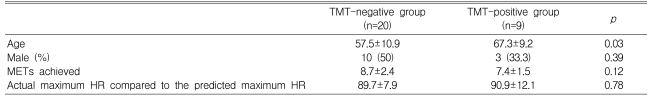
During the exercise ECG test, the metabolic equivalents (METs) achieved were 7.4±1.7 in the CAD group and 8.4±2.4 in the non-CAD group (p=0.22) (Table 2). Eight patients (57.1%) in the CAD group and 18 patients (69.2%) in the non-CAD group (p=0.18) achieved ≥ 85% of the predicted maximum HR. The proportion of patients who achieved ≥ 85% of the predicted maximum HR or a positive ECG change during exercise, which constituted sufficient stress to determine the ischemic response, did not differ between the CAD and non-CAD groups (71.4 vs. 73.1%, respectively, p=0.91) (Table 2). Positive exercise ECG tests (TMT positive) were obtained in eight patients (57.1%) in the CAD group, but in only one patient (3.8%) in the non-CAD group (p=0.001). Twenty patients (50%) had negative results on the exercise ECG test (TMT negative, Table 3).
Laboratory Results
Serum IMA level
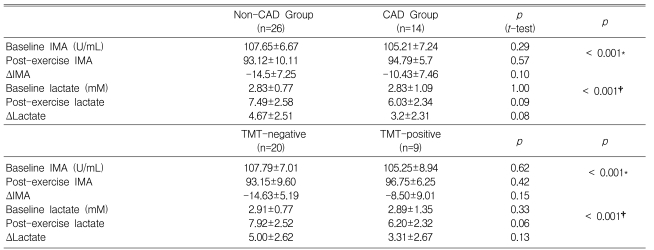
The baseline and post-exercise IMA values did not differ significantly between the CAD and non-CAD groups (105.2±7.2 vs. 107.7±6.7 U/mL at baseline and 93.1±10.1 vs. 94.8±5.7 U/mL post-exercise, p=0.29 and 0.57, respectively; Table 4 and Figure 1). The changes in IMA after exercise was not different between the CAD or non-CAD groups (-10.4±7.5 vs. -14.0±7.6 U/mL, p=0.10) (Figure 1). In only two cases (5%), did the post-exercise IMA level increase compared with the baseline value. The serum IMA concentration was also analyzed according to the results of the treadmill exercise ECG test. The baseline serum IMA concentrations did not differ between the TMT negative (n=20) and positive (n=9) groups (107.79±7.01 vs. 105.25±8.94, p=0.62) (Table 4). Neither did the post-exercise IMA values (93.15±9.60 vs. 96.75±6.25 mM in the TMT negative and positive groups, p=0.42). The change in the IMA concentration during exercise was not different either (-14.63±5.19 vs. -8.50±9.01 mM in the respective groups, p=0.15) (Table 4, Figure 2).
Serum lactate level
The baseline and post-exercise lactate levels did not differ significantly between the CAD and non-CAD groups (2.8±1.1 vs. 2.8±0.8 mM at baseline and 7.5±2.6 vs. 6.0±2.3 mM post-exercise, p=0.29 and 0.51, respectively) (Table 4, Figure 1). The changes in the lactate level after exercise did not differ either (3.2±2.3 vs. 4.7±2.5 U/mL, p=0.08). When the serum lactate values were analyzed according to the exercise ECG test results, the baseline and post-exercise lactate levels did not differ significantly between the TMT-positive and -negative groups (Table 4).
Correlation between the serum IMA and lactate levels
The serum IMA level was strongly correlated with the serum lactate level (r=-0.71, p<0.01). The change in the IMA level was also significantly correlated with the change in the serum lactate level (r=-0.61, p<0.01) (Figure 3).
DISCUSSION
IMA is produced by free radicals from albumin when the myocardium is in an ischemic state2, 16). This has been validated by several studies using human ischemic myocardial models with transient balloon occlusion of the coronary artery during PCI8-10). In fact, the serum IMA level is so sensitive to myocardial ischemia that the level increases even when the level of troponin stays within normal limit. This means that IMA can be used as a marker of reversible myocardial ischemia. According to a previous report8), immediately following 2-3 min of balloon occlusion of the coronary artery, 18 of 19 patients (94.7%) showed elevated levels of IMA. Several studies have also suggested that if the IMA level is considered with concomitant ECG changes in the case of acute coronary syndrome, the diagnostic accuracy rate may be enhanced. According to some studies, measurement of IMA can be used at admission, in combination with measurement of cardiac troponin and ECG, to confirm or exclude a final diagnosis of ischemic heart disease and to exclude myocardial infarction1, 7).
While minimal-effort or resting chest pain remains the main complaint in patients with acute coronary syndrome, effort-related chest pain is the main symptom of stable angina. However, the usefulness of IMA in identifying effort-related myocardial ischemia was unclear until a number of recent studies reported no specific relationship between IMA plasma levels and coronary ischemia during exercise13, 14). In fact, several studies have cast doubt on the usefulness of plasma IMA in the setting of myocardial ischemia during exercise. A recent report14) showed that the IMA level was significantly reduced during exercise, and it was suggested that hemoconcentration with a subsequent increase in the plasma albumin level during exercise was responsible for the decrease in the non-bound portion at a fixed level of cobalt stress. It has also been suggested that the elevated plasma IMA levels seen during myocardial ischemia are ultimately caused by other mechanisms, such as hemoconcentration13). We also found that the serum IMA level dropped significantly right after exercise in both groups, which is consistent with these previous results13,14). When the change in the serum IMA level (ΔIMA) was analyzed according to the results of the exercise ECG test, ΔIMA during exercise did not differ significantly between the TMT positive and negative groups. Similarly, it was reported that there was no interaction between ΔIMA and the results of a stress test13). In that study, blood samples were collected before the stress test (baseline), at the peak of exercise, and 60 min after completion of the exercise test. In contrast, we collected blood samples before the application of stress and 5 min after the exercise ECG test. Even with this difference in blood sampling, we obtained the same results regarding the response of serum IMA to exercise. This implies that the serum IMA level is not an useful marker of ischemia in patients with stable angina. Another study also suggested that the elevation of lactate level during the exercise may influence the serum IMA level, which could account for reduced serum IMA level after exercise17). Consistent with these studies, the serum lactate level in our study showed an excellent correlation with the serum IMA level. However, since our study was not designed to elucidate the exact mechanism of the response of IMA to exercise-induced myocardial ischemia, our result showing a relationship between lactate and IMA should be used only to verify the report of Zapico-Muniz17). In conclusion, no other diagnostic usefulness of the IMA level could be found in the setting of symptom-limited exercise. These findings may support the caution for IMA to be used to determine ischemia in patients with stable angina.
Study Limitations
This study has several limitations. First, the number of patients studied was relatively small. Second, in the symptom-limited exercise ECG test, 26.9 and 28.6% of the patients in the non-CAD and CAD groups, respectively, did not reach a sufficient exercise level (≥ 85% of the maximum HR) or ECG change during the exercise period, meaning that a portion of the CAD group may not have developed exercise-induced myocardial ischemia, which could have affected our results. Therefore, our results should be applied only to symptom-limited exercise stress tests; moreover, additional studies with more patients are needed to validate the meaning of the IMA level in terms of myocardial ischemia during exercise. Third, in the TMT positive and negative groups, patient age was not matched between the two groups, although the parameters of sex, METs achieved, and achieved HR over the predicted maximum HR (%) were matched. This could have affected our results. Fourth, we did not examine the change in IMA long after exercise (e.g., 1 h later). Instead, we considered only two time points (before exercise and 5 min after exercise), which means that the time window of this study might not be long enough to reveal the entire effect of exercise on the IMA plasma level. Finally, if we had analyzed other biochemical parameters to elucidate the underlying mechanism of IMA change during exercise, we might have been able to suggest a possible mechanism using statistical approaches, such as regression analysis. Further study is needed to better understand this point.
Notes
This study was supported by the Cardiovascular Research Foundation, Seoul, Korea.