Ultrastructure of Neuromuscular Junction in Vacor-induced Diabetic Rats
Article information
Abstract
Objectives
Rodenticide Vacor causes a severe peripheral neuropathy in humans.
Electrophysiologic studies on a peripheral motor nerve-skeletal system of Vacor-treated rat showed decreased amplitude of muscle action potential without conduction velocity abnormalities. The ultrastructural studies of the neuromuscular junction were performed to clarify the anatomic site of the Vacor-induced peripheral neuropathy in male Wistar rats.
Methods
After oral administration of a single dose of Vacor, 80 mg/kg of body weight, to the experimental animals, neuromuscular junctions within the interosseous muscles of the hind foot were observed in time.
Results
No axon terminal change was noted until 24 hours after the administration of Vacor. Remarkable loss of presynaptic vesicles and swollen endoplasmic reticulum in the axon terminal were developed at 3 days after Vacor treatment. Progressive degenerative changes consisting of marked loss of presynaptic vesicles, focal disruption of membrane in the axon terminal with disappearance of the number of the damaged axon terminal appeared, and flattening of postsynaptic folds was also seen.
Conclusions
These results suggest that degenerative changes in axon terminal at neuromuscular junction may contribute to the peripheral neuropathy developed in the early phase of Vacor poisoning.
INTRODUCTION
Vacor (N-3-pyridylmethyl-N′-p-nitrophenylurea)-poisoned patients had complained of various neurological symptoms such as urinary retention, burning sensation of the foot, areflexia and orthostatic hypotension1–4). Various neurotoxicities of Vacor were reported in the acute toxic phase. However, the cause of neurotoxicity is not well known5).
In the electrophysiologic studies of the peripheral nerve, Vacor did not affect the conduction velocity. However, the decrease of action potential amplitudes was developed on the sciatic-tibial nerve of a Vacor-treated rat6). The decrement of action potential amplitude indicates that axonal or muscular dysfunction might be induced by Vacor.
To define these electrophysiologic abnormalities in the Vacor-induced peripheral neuropathy, an ultrastructural study was carried out in the neuromuscular junction of the interosseous muscle in Vacor-treated rats.
MATERIAL AND METHODS
Male Wistar rats (n=30), weighing 200–250g, were used in this study. A single dose of Vacor (N-3-pyridylmethyl-N′-p-nitrophenylurea, MW 272), 80 mg/kg of body weight, was administrated by an orogastric canule, while distilled water was given in controls.
The experimental animals were sacrificed at 1, 3, 7, 14 days after administration of Vacor. The animals were perfused through the jugular vein with 5% glutaraldehyde in 0.1 M sodium phosphate buffer.
To examine the morphological changes of the neuromuscular junction, tissues were taken from the interosseous muscles of the hind foot.
The tissue fragments were divided into small blocks, 2×2×1 mm each, and subsequently immersed in 2.5% glutaraldehyde for 12 hours at 10 °C. They were postfixed in 2% osmium tetroxide for 4 hours at room temperature. After dehydration in graded ethanol, they were embedded in epon mixture for three days at 50 °C.
Thick sections, 1 μm in thickness, stained with toluidine blue were prepared for light microscopy. Thin sections, 80 nm in thickness, were obtained from the representa-tive areas and stained with uranyl acetate and lead citrate. The sections were examined with a Zeol 100 CXII electron microscope.
RESULTS
In control rats, myelinated axons and axon terminals at neuromuscular junctions of the hind foot interosseous muscle maintained their structural integrity well. No degenerative changes were seen in myelinated nerve fibers (Fig. 1). The axon terminal contained mitochondria, concentrically lamellated endoplasmic reticulum (ER) and numerous small round presynaptic vesicles (Fig. 2), and closely contacted to sarcoplasmic membrane of myocyte.
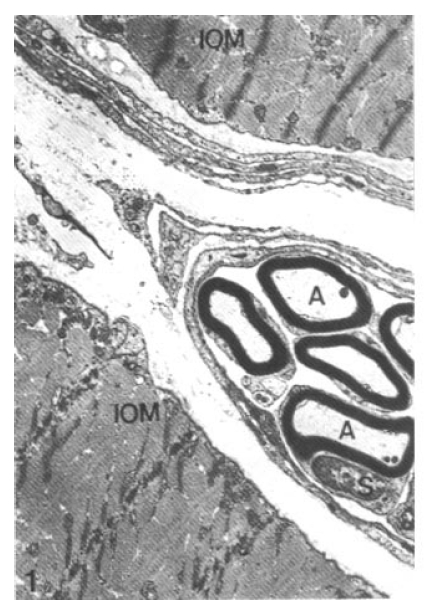
Normal posterior tibial nerve. Note intact myelinated axons (A) and Schwann cell (S) between interosseous muscle (IOM) of the hind foot (× 11,200).

Normal neuromuscular junction. Note the axon terminal filled with many presynaptic vesicles (SV), mitochondria (M), and lamellated endoplasmic reticulum (ER) (× 32,000).
Morphological change of the axon terminals in neuromuscular junctions was not evident within one day after Vacor administration (Fig. 3). The synaptic cleft between the axon terminal and postsynaptic folds of sarcoplasm was not widened. At 3 days after Vacor administration, there was remarkable loss of presynaptic vesicles and swollen ER in the axon terminal (Fig. 4).
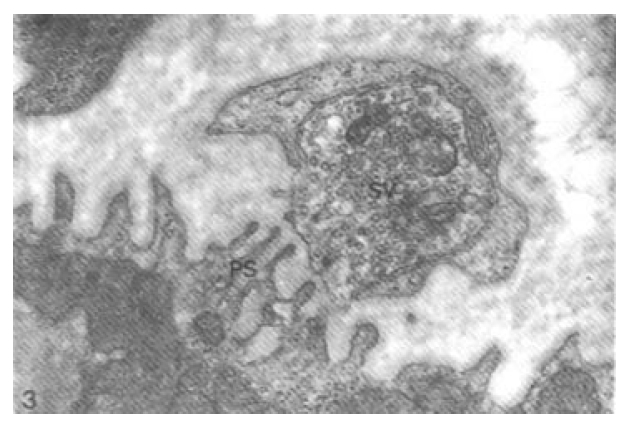
Neuromuscular junction one day after Vacor administration (80 mg/kg). No degenerative change is seen. Note intact axon terminal containing many presynaptic vesicles (SV), synaptic cleft and delicate papillary postsynaptic folds (PS) of sarcoplasm (× 88,000).
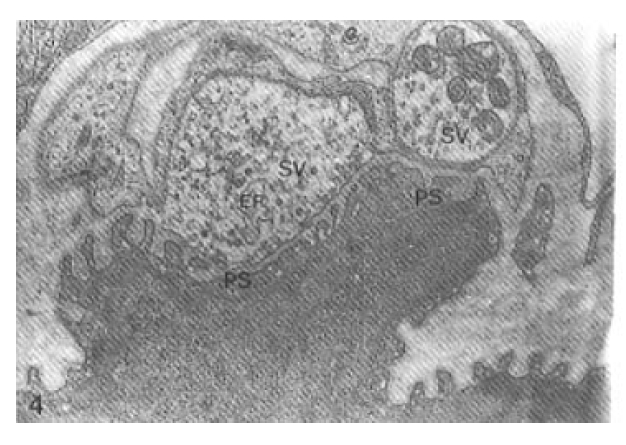
Neuromuscular junction days after Vacor administration. Note mild degenerative changes consisting of significantly reduced number of presynaptic vesicles (SV) and dilatation of endoplasmic reticlum (ER) in the axon terminal, and flattening of postsynaptic folds (PS) (× 52,000).
The papillary postsynaptic folds were partially flattened. Progressively decreased number of presynaptic vesicles and disruption of membrane of the axon terminal with myeloid body formation were seen at 7 days after Vacor administration (Fig. 5). The synaptic cleft was widened. The axon terminals significantly reduced in number at 14 days after Vacor administration. A few presynaptic vesicles were engulfed in irregular Schwann cell processes identified by a delicate lining of their basal lamina (Fig. 6).

Neuromuscular junction 7 days after Vacor administration. Note progressive degenerative changes consisting of marked loss of presynaptic vesicles (SV) and focal disruption of membrane (arrow) in the axon terminal with myeloid body (MB) formation and flattened postsynaptic folds (PS) (× 52,000).
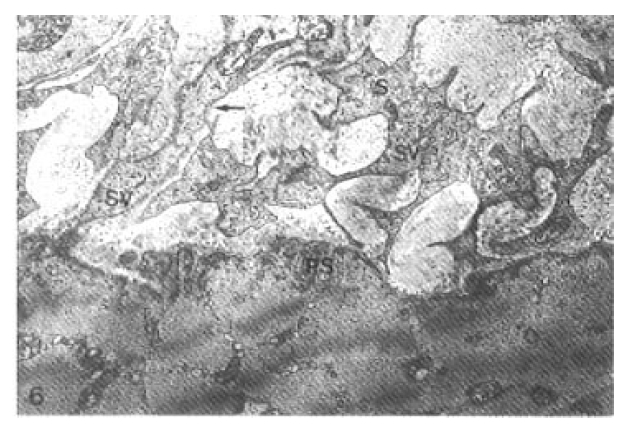
Neuromuscular junction 14 days after Vacor administration. Some presynaptic vesicles were disappeared, and the lesion is replaced by irregularly elongated processes of Schwann cell (S) which engulfed a few presynaptic vesicles (SV). Note thin delicate basal lamina along the processes (arrow). Postsynaptic folds (PS) were simplified (× 22,400).
Some axon terminals had only a few synaptic vesicles, and postsynaptic folds were markedly flattened and simplified (Fig. 7).
DISSCUSSION
Degenerative changes and segmental demyelinations were developed in diabetic neuropathy with long duration.
In the acute phase of experimental diabetes induced by streptozotocin, pathologic changes of the nervous system were not found, and it required more days or weeks to develop.
The diabetes induced by Vacor has various neurological complications such as urinary retention, burning sensation of the foot, areflexia and orthostatic hypotension, etc1–4,7,8). Lee et al.5) had reported that rats given Vacor show various morphological changes such as necrosis of axon, myelin sheaths and glia cells in the spinal cord with unchanged organelles of the peripheral nerves, but its correct mechanism is not clear.
Park6) reported that the amplitude of the compound muscle action potential decreased but conduction velocity of sciatic-tibial nerve of a Vacor treated rat was unchanged, and Na+, K+-ATPase activity and inositol content of sciatic nerve were unchanged, which were different from those of streptozotocin diabetes.
However, neurotoxicity was reported in the acute toxic phase of Vacor poisoning. Lewitt9) reported the active breakdown of myelin sheath in a streptozotocin diabetic animal.
In this study morphological changes of axon terminals at neuromuscular junction were not evident within one day after Vacor treatment. However, loss of presynaptic vesicles in axon terminal and swollen endoplasmic reticulum were developed at 3 days after Vacor administration. These abnormalities of axon terminal progressed at 7 and 14 days after Vacor treatment.
These findings may provide us the evidence that decreased amplitude of compound muscle action potential caused by axonal degeneration.
