Role of specific IgE, IgG and IgG4 antibodies to corn dust in exposed workers
Article information
Abstract
Background & Methods
To evaluate the role of specific antibodies to corn dust (CD) and their relationship to respiratory dysfunction, we detected serum specific IgE(sIgE), IgG(sIgG) and IgG4(sIgG4) antibodies by ELISA in 42 employees working in the animal feed industry and 27 unexposed controls.
Results
Our survey revealed that 15 (34.9%) subjects had work-related respiratory dysfunction associated with or without nasal symptoms. Among these subjects, eight had airway hyper-responsiveness to methacholine. Significant differences were noted in sIgE and sIgG4 between exposed and unexposed groups (p=0.04, p=0.00 respectively), but no difference was noted in sIgG (p=0.1). Although there was no significant difference in the prevalence of specific IgE antibody between symptomatic (29%) and asymptomatic groups (19%, p=0.55), the specific IgE levels were significantly higher in symptomatic workers than in asymptomatic workers (p=0.03). Specific IgG antibody was detected in 1(6%) symptomatic and 4(15%) asymptomatic workers (p=0.46). Specific IgG4 antibody was detected in 11(73%) of symptomatic and 21(78%) of asymptomatic workers (p=0.90). The higher prevalence of sIgG4 antibody was noted in workers with sIgE antibody (p=0.001). The correlation between sIgG and exposure duration was significant (r=0.36, p=0.02). There was no association between the prevalence of sIgE, sIgG, and sIgG4 to exposure intensity, smoking or atopic status.
Conclusion
These results suggested that the existence of sIgG and sIgG4 might represent a response to CD exposure, and that some unexposed subjects had sIgG to CD. Specific IgE might play a role in the development of respiratory symptoms.
INTRODUCTION
Chronic inhalation of grain dust has been shown to cause acute and chronic airway injury characterized by bronchitis and airflow obstruction1–4). Longitudinal studies have shown accelerated deterioration of pulmonary function in these grain dust workers5), the severity of which appears to be related to the concentration of airborne grain dust in the work environment4, 6). In regard to pathogenetic mechanism of corn dust-induced asthma, our previous report demonstrated that inhalation of corn dust (CD) could induce IgE-mediated bronchoconstriction7). However, there have been a few studies suggesting that endotoxin included in the CD might induce airway inflammation, not via immunologic mechanism8, 10). Further studies are needed to determine the role of specific IgG in occupational asthma studies. Our previous study dealing with grain dust-induced occupational asthma11) showed that only three of six patients had high specific IgE antibodies to grain dust, while all had high specific IgG antibodies, which suggested that sIgG might represent exposure to grain dust.
In order to evaluate the clinical significance of serum sIgE, sIgG and sIgG4 antibodies and their relationships to respiratory dysfunction in CD-induced asthma, we studied the prevalence of CD-specific IgE, IgG and IgG4 antibodies by ELISA in 43 CD-exposed workers. The relationship of sIgE, sIgG and IgG4 antibodies was also investigated.
MATERIAL AND METHODS
Subjects
All of the 42 subjects exposed to CD were male and worked for the Dongbang feed industry in Suwon, Korea. Of these employees, 31 were process workers who mixed the materials as well as carried them. They were classified as group II (intermediate exposure, n=12) and group III (high exposure, n=19) according to exposure intensity which was measured by a dust air sampler (Gilian INS, U.S.A.). Twelve employees were office workers and were classified as group I (low exposure group). Lower respiratory symptoms referred to cough, sputum, chest tightness or shortness of breath. Symptomatic employees were those workers who had experienced lower respiratory symptoms during and after CD exposure. Atopy was defined as a positive reactor to more than one of the common inhalant allergens on the skin prick test12). All the subjects gave their informed consent as regulated by Ajou University Hospital.
Sera
Sera from 43 employees were collected and stored at −20°C, as well as sera from control subjects consisting of 27 individuals who had never been exposed to CD, and who had demonstrated negative skin tests to 50 common inhalant allergens including CD extracts.
Preparation of extracts
CD was obtained from the patients’ workplace. It was extracted with phosphate-buffered saline [(PBS, pH 7.5), 1: 5 w/v] at 4°C for 1 h followed by centrifugation at 5,000 rpm. The supernatant was dialyzed (the cut-off molecular weight was 6,000 Da) against 4 litres of distilled water at 4°C for 48 h, passed through the filter (0.2 μm pore sized) to exclude bacterial contamination, and lyophilized at −70°C for the preparation of antigens used in ELISA.
ELISA
ELISA was performed according to the previously described method8). A 96-well EIA flat-bottomed plate (Dynatec, USA) was filled with 10 μg/well CD antigens in a carbonate buffer (pH 9.6), and coated with the buffer only, which was preliminarily determined as the optimal concentration. After overnight incubation at 4°C, the plates were washed three times with 0.05M Tween-phosphate-buffered saline (PBST). To each well was added 250 μl of 5% bovine serum albumin (BSA)-PBST, which was then incubated for 60 minutes at 37°C. All three assays were performed in triplicate.
Anti-com dust IgG ELISA
Fifty microlitres of diluted patients’ serum or negative control serum (1/200 in diluent buffer; PBST containing 3% BSA) were added to each well coated with CD. After incubation for 2 hours at 37°C, the wells were washed three times with PBST. One hundred microlitres of horseradish peroxidase (HRP)-conjugated goat antihuman IgG (Sigma Co. USA) diluted into 1/2000 v/v with 3% BSA-PBST, were added to each well. The plates were then incubated at 4°C for 2 hours. The wells were washed three times with PBST and then 50 μl of substrate solution were added, containing 0.01M O-phenyl deamine-HCl in citrate phosphate buffer, pH 4.2, supplemented with 0.012% H2O2 before use. After incubation for 15 minutes at room temperature, 50 μl of 1 N H2SO4 were added to stop the reaction. The optical density of the solution at 490 nm was determined with a microtitre plate reader (MR 600, Dynamic Product, USA). The antibody titres were expressed as absorbances at 490 nm. The positive cut-off value was determined from the mean and three-fold standard deviation of the 27 negative controls. The final absorbances were obtained by the subtraction of the absorbance of each uncoated well.
Anti-corn dust IgG4 ELISA
Fifty microlitres of patient serum or negative control serum (undiluted) were added to each well coated with 10 g/well of corn dust, and incubated for 2 hours at room temperature. After the wells were washed three times with PBST, 50 μl of biotin-conjugated mouse monoclonal anti-human IgG4 (Sigma Chemical Co., USA) was diluted to 1/1000 (v/v) with 5% BSA-PBST and were incubated for 2 hours at 37°C. The wells were washed three times with PBST. Then, 50 μl of 1/1000 diluted streptavidin-horseradish peroxidase (Sigma Chemical Co. USA) were added and incubated for 30 minutes. The wells were washed three times and 50 μl of substrate solution (55 mg of 2,2′-azido-di-3 ethylbenzthiazoline sulphonic acid; Sigma Chemical Co.) in 100 ml of 70 mM citrate phosphate buffer were added to each well. After incubation for 5 min, 50 μl of 2 mM sodium azide were added to stop the reaction. The absorbance was measured at 410 nm with a microplate reader, and the antibody titres were expressed as absorbance values, The positive cut-off value was determined from the mean absorbance and 3-fold standard deviations of the 27 controls.
ELISA for specific IgE antibodies to corn dust extracts
The wells were incubated for 2 hours at room temperature with 50 μl of either the patients’ sera (undiluted) or control sera from the 27 controls. After washing three times with PBST, 100 μl of the 1: 1000 v/v biotin-labeled goat anti-human IgE antibody (Sigma Co. USA) were added to the wells and incubated for 2 hours at room temperature. The wells were then washed three times with PBST and incubated with 1: 1000 v/v streptavidin-peroxidase (Sigma Co. USA) for 30 minutes before another washing step, which was followed by incubation with 50 μl ABTS (2.2′-azinobis-3-ethyl-benzthiazoline sulfuric acid in a citrate phosphate buffer) for 10 min at room temperature. The reaction was stopped by the addition of 50 μl of 2 N sodium azide, and the absorbance was read at 410 nm by an automated microplate reader. The positive cut-off value was determined from the mean and 3-fold standard deviation of the controls.
Statistical analysis
The x2 ANOVA tests were applied using the SPSS version 7.0 (Chicago) to evaluate the statistical differences among the data: A p value of 0.05 or less was regarded as significant.
RESULTS
Immunologic studies
Figures 1 and 2 illustrate the distributions of sIgE, sIgG and sIgG4 antibodies to CD in all employees and controls. Significant differences were noted in sIgE and sIgG4 among the four groups (p=0.04, p=0.01), with no significant differences in specific IgG among the four groups (p=0.21) because unexposed controls had high specific IgG to CD. Table 1 summarizes the prevalence of specific IgE, sIgG, and sIgG4 antibodies to CD. There were no associations between the presence of respiratory symptoms and the prevalences of sIgE, sIgG and sIgG4 antibodies. Of the 15 symptomatic workers, 4 (27%) had high specific IgE bindings, whereas 10 (37%) out of 27 asymptomatic workers were positive (p=0.55). One (6%) out of 15 symptomatic workers had sIgG, and 11 (73%) had sIgG4 antibodies to CD (p=0.46, p=0.90, respectively). When comparing specific antibody levels between symptomatic and asymptomatic groups, no significant differences were noted in specific IgE level (p=0.55), sIgG and sIgG4 levels as shown in Table 1 (p=0.46, 0.90, respectively). An appreciable number of asymptomatic workers also had sIgG4 (78%) or sIgG (15%) antibodies. The prevalence of sIgG4 antibody was significantly higher in workers with sIgE (p=0.001), and not in those with sIgG (p=0.57) as shown in Table 2.

Comparison of serum specific IgE antibody to corn dust in 43 exposed workers and unexposed controls. I: Low exposure group, II: Intermediate exposure group, III: high exposure group.

Comparison of serum specific IgG and IgG4 antibodies to corn dust in 43 exposed workers and unexposed controls. I: Low exposure, II: Intermediate exposure, III: High exposure
Relationship between intensity and length of exposure and serum specific antibodies to corn dust
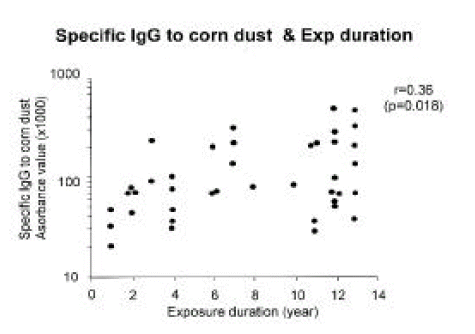
In this study, 42 workers were classified into three groups according to the exposure intensity of their workplace. The prevalences of sIgG and sIgG4 were not significantly different among the three different exposure groups. The length of exposure varied from 1 to 13 years. There was a significant correlation between duration of CD exposure and sIgG level, but not with sIgG4 level [r=0.36 with sIgG (p=0.02 in Fig 3), r= 0.28 with sIgG4 (p=0.07), data was not shown].
Association between smoking and atopy, and specific IgG and IgG4 antibodies
Table 3 and 4 show that there is no association between atopy and the prevalence of sIgE, sIgG and sIgG4 antibodies (p=0.09, 0.71, 0.52, respectively). Table 3 and 5 show a significant association between smoking status and the prevalence of sIgG4 (p=0.04), but not with sIgE (p=0.49) nor sIgG (p=0.12) antibodies.
Comparison of skin test reactivity to common inhalant allergens
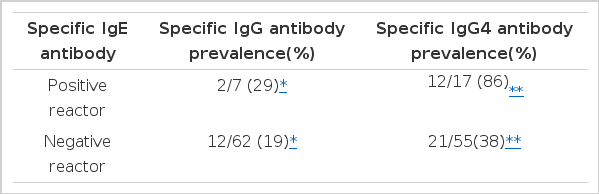
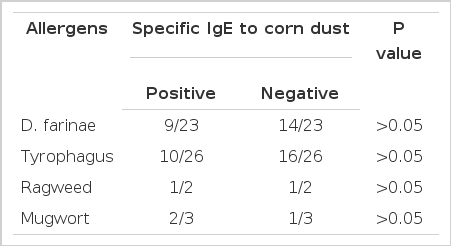
Table 6 shows comparison of skin reactivity to other common inhalant allergens between positive and negative reactors to CD on skin prick test. No significant associations were found with skin reactivities to D. farinae, Tyrophagus spp., ragweed or mugwort pollen antigens (p>0.05).
DISCUSSION
A previous study7) demonstrated the presence of serum specific IgE antibodies to CD by ELISA and identified the IgE-binding components within CD extract by immunoblotting in the sera of asthmatic workers. In this study, significant differences were noted in sIgE antibody between exposed and unexposed groups. Moreover, the specific IgE antibody level was significantly higher in symptomatic workers than in asymptomatic workers. These findings support the view that specific IgE to CD may be responsible for asthmatic symptoms in exposed workers. However, some asymptomatic workers had high specific IgE antibody. Further studies will be needed to investigate the clinical course of asymptomatic sensitizers.
There have been a few studies suggesting inhalant allergens, such as house dust mite, pollens, fungus or storage mites included in grain dust, could act as allergens instead of grain dust itself8). Several other investigators9, 10) reported that endotoxin included in CD extract might induce airway inflammation. However, our previous study11) demonstrated six asthmatic subjects who had worked in the same workplace, showed negative bronchial challenges on grain dust-bronchoprovocation test, in contrast with six asthmatic subjects with positive bronchial challenges. In the present study, the skin prick test results to other common inhalant allergens showed no significant associations with skin reactivity to CD. Furthermore, CD-ELISA inhibition test results showed minimal inhibitions by house dust mite or mugwort pollen allergens, which were the most prevalent antigens on skin prick in 43 employees tested. These findings suggest that CD used in the present study could act as an allergen itself. The possibility of contamination by other inhalant allergens or cross-reactivity with them seems to be extremely low.
The sIgG response in occupational asthma studies seems to be complex, and could elicit a variable response depending on the kind of antigens involved13–15). In this study, we succeeded in detecting sIgG and sIgG4 antibodies to CD by ELISA. ELISA inhibition tests with serial addition of various concentrations of CD antigens showed significant inhibitions in a dose-dependent manner, not by other inhalant allergens (data was not shown) suggesting that antibodies detected in this ELISA system might be derived from specific bindings. In the case of isocyanate-induced occupational asthma studies, the level of sIgG to hexamethylene diisocyanate and methylene diisocyanate bore a satisfactory association with the results of specific inhalation challenges, while the levels of specific IgE did not16). Our previous study17) on reactive dye-asthma revealed that there was an association between work-related respiratory symptoms and the prevalence of sIgG or sIgG4. However, several studies in other occupational settings have suggested that the presence of sIgG may represent a response to high dose exposure, not directly related with the development of respiratory symptoms18). Quirce et al.19) reported that all employees at a carmine dye factory, regardless of their occupation or presence of respiratory symptoms, had high levels of sIgG, probably as a consequence of the highly carmine-contaminated environment to which they were exposed. Similarly, the study of sIgG in workers of the potato-processing industry20) demonstrated that sIgG was found in nearly all workers, and a specific IgG4 subclass was found in about half of the workers, suggesting IgG4 as a predominant antibody. In this study, most workers, regardless of presence of respiratory symptoms, had sIgE, sIgG, sIgG4 antibodies when exposed to CD, and no association was found with the presence of respiratory symptoms. The correlation between length of exposure and sIgG was significant. These results suggest that the existence of sIgG and sIgG4 to CD might represent a response to CD exposure without being related to respiratory symptoms.
This study revealed no association between smoking and sIgE or sIgG antibodies to CD. However, a significant association was found between sIgG4 antibody and smoking status. Also, no association was found between atopy and sIgG or sIgG4 antibodies, which was a comparable finding to those in other studies21), suggesting that pre-existing atopy did not affect the development of sIgE, sIgG and sIgG4 antibodies to CD. Further investigations will be needed to study the role of smoking in the development of sIgG4 antibody to CD.
Our results suggest that CD could induce an immunologic, IgE-mediated response in exposed workers, which may contribute to the development of respiratory symptoms. The existence of the serum specific IgG and IgG4 antibodies to CD might represent exposure to CD. This response, however, is probably of no relevance to the occurrence of respiratory symptoms.
Acknowledgements
This manuscript was funded by Ajou Post-graduate School of Medicine (1996).