A Case of Behçet’s Disease with Superior and Inferior Vena Caval Occlusion
Article information
Abstract
Behçet’s disease is a chronic multisystemic disorder involving many organs and characterized by recurrent oral and genital ulcers and relapsing iritis. A case of BD with large vein thrombosis involving superior and inferior vena cava is presented. Large vein thrombosis in BD is not commonly developed and most commonly observed in the inferior or superior vena cava. A review of the literature emphasizes the rarity of the combined superior and inferior vena caval occlusion. Existence of extensive large vein occlusion in BD is associated with limited therapy and poor prognosis.
INTRODUCTION
Behçet’s disease (BD) is a multisystem vasculitis, probably autoimmune in origin. It is characterized by systemic organ involvement, including mucocutaneous, ophthalmic, neurologic, cardiovascular, pulmonary, urogenital, gastrointestinal, musculoskeletal system. Vascular involvement in BD is not uncommon and closely related with the prognosis of BD. BD associated with large vascular lesions is called “Angio-Behçet’s syndrome”. Venous occlusions are frequently observed, most commonly in the inferior or superior vena cava1). Occlusion of both the superior and inferior vena cava was seen and reported1,2).
The treatment of BD with vascular lesions remains empirical and palliative, but usually resistant to all types of treatment. In cases of venous occlusion, the prognosis is relatively favorable due to the development of collateral veins3). More extensive venous occlusions have further limited therapy and less favorable outcome4). To avoid serious vascular complications in BD, early detection and treatment of peripheral vein thrombosis are necessary for preventing propagation of the thrombus.
CASE REPORT
A 26-year-old woman was admitted for dyspnea, coughing and generalized edema for the last 5 months. She had a symptoms complex of recurrent oral aphtous ulcer, genital ulcer, erythema nodosum like skin lesions, uveitis and positive Pathergy test, and was diagnosed to have BD according to the diagnostic criteria proposed by the International Study Group for Behçet’s disease5). She gave no history of smoking, consumption of alcohol or any drugs. Two years ago, bypass surgery was performed due to deep vein thrombosis of left lower extremity (Fig. 1, 2). At that time, she did not follow-up and was not prescribed for any medication. On physical examination, the patient was uncomfortable and slightly pale. She became dyspneic on supine for the examination. No rash or lymphadenopathy was found. There was marked edema over the face, neck, both shoulders, anterior chest and lower extremities. Also, collateral vessels formation in the anterior chest wall and abdomen were noticed. Diminished breath sounds were present over both lung fields. The abdomen was distended and non-tender, but a fluid wave and shifting dullness were present. The liver was palpable 5 cm below the costal margin, but the spleen was not felt and no masses were palpable.

Venography of left lower extremity. Left iliac vein is obliterated and multiple collateral veins (arrow) are shown.
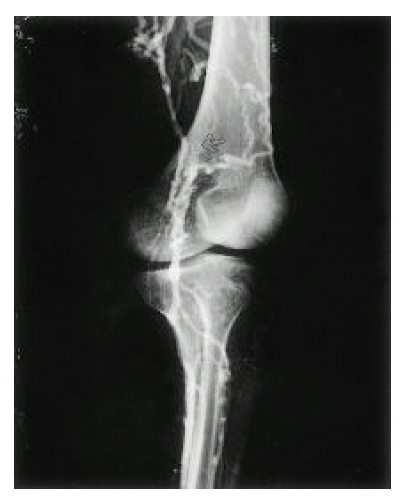
Venography of left lower extremity. Popliteal vein is abruptly obliterated (arrow) and superficial femoral vein is not opacified. Multiple collateral vessels are shown.
Blood tests showed that the white blood cell count was 10,600 per cubic millimeter, hemoglobin 11.7 g/dL, hematocrit was 33 percent, and the platelet count was 45,000 per cubic millimeter. The erythrocyte sedimentation rate (ESR) was 35 mm/hour. The values for blood chemistries were all within the normal limits. She had no family history of clotting abnormalities and the routine coagulation tests (prothrombin time and activated partial-thromboplastin times, bleeding time) and protein C, protein S, antithrombin III levels were within normal range. Antinuclear antibody (ANA), anticardiolipin antibody, lupus anticoagulant and rheumatoid factor (RF) were negative. C3, C4 were within normal limits. IgG, IgA and IgM were 587 mg/dl, 154 mg/dl and 214mg/dl, respectively. The pleural fluid showed characteristic findings of transudate.
An X-ray film of the chest showed bilateral pleural effusion. Computed tomographic (CT) scan of the thorax (Fig. 3) demonstrated large amounts of bilateral pleural effusion and SVC occlusion with prominent collateral vessels at the chest wall, both axillary areas and within the mediastinum. Abdominal CT (Fig. 4) showed ascites and obliterated IVC, except for the short segment near the heart. Also, many collateral vessels were noted in the splenic hilar area, hepatosplenic ligament, retroperitoneum, mesentery, both perihilar areas of kidney, both paravertebral areas, perirectal area and the whole abdominal wall. Multiple round and wedge shaped, non-enhancing hypodense lesions suggest multiple liver infarction and diffuse hepatomegaly and mild splenomegaly were noted.
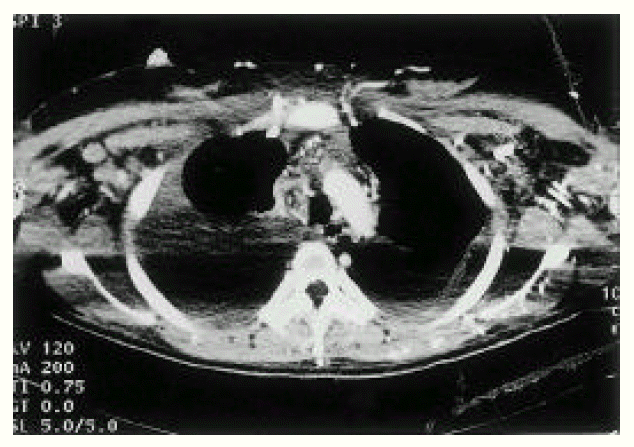
CT of chest. SVC and brachiocephalic veins are obliterated (arrow) and multiple collateral vessels are opacified at anterior chest wall and both axilla.
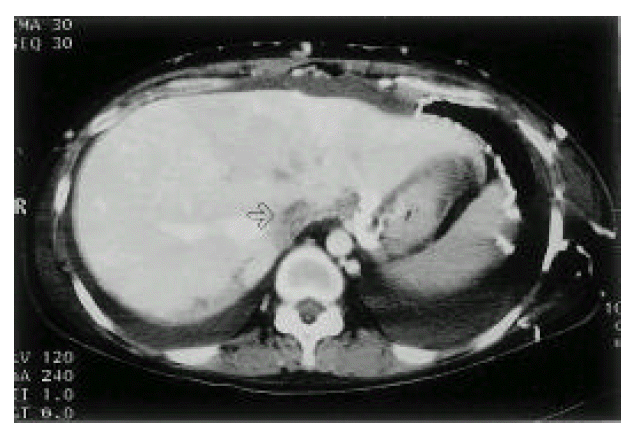
Abdominal CT. IVC is filled with low attenuate thrombus (arrow). Multiple collaterals are noted at the anterior abdominal wall, hepatogastric and hepatosplenic ligament area.
The patient was treated with prednisolone, diuretics (furosemide) and anticoagulant and antiplatelet agents. Intermittent therapeutic thoracentesis of both pleural effusions were done. However, both pleural effusions were not improved. So, chest tube insertion was performed for drainage of refractory pleural effusion. Thereafter, her general condition did not change and dyspnea was slowly progressed and aggravated. Hypotension, anuria and shock developed and progressed despite medical supportive therapy, and the patient died.
DISCUSSION
Behçet’s disease is a multisystem vasculitis with mucocutaneous, ophthalmic, neurologic, cardiovascular, pulmonary, urogenital, gastrointestinal, musculoskeletal involvement and other features. Vascular involvement in BD is not uncommon, with an overall incidence ranging from 7% to 60%, and any large or small artery, vein or organ may be involved in an unpredictable combination6). Vascular involvement in BD is seen more frequently in male patients7). BD associated with large vascular lesions is called “Angio-Behçet’s syndrome” and four types of vascular lesions are recognized: arterial occlusion, aneurysms, venous occlusion and varices. Vascular lesions more commonly involve the venous system than the arterial systems (incidence of venous system involvement between 18–24% in BD)8).
The most frequent presentation of venous lesion of BD is superficial thrombophlebitis and thrombosis9). Venous occlusions are frequently observed, most commonly in the inferior or superior vena cava1). Thrombosis of the superior or inferior vena cava in BD has been recognized and frequently reported10,11) The incidence of superior and inferior vena caval occulsions reported in the literature are 9.0% and 2.5%, respectively, and they manifested as superior vena cava syndrome or lower limb edema or would be life-threatening if not treated promptly2). Occlusion of both the superior and inferior vena cavae was seen and reported12).
The common underlying histopathologic lesion in BD is nonspecific vasculitis which could involve any systemic vessel. The pathologic process in the wall of the blood vessels in BD is a result of immune complex deposition, leading to complement fixation and ploymorphonuclear leukocyte activation6). The characteristics of vasculitis are perivascular infiltrations of inflammatory cells, proliferation of vascular endothelial cells, some tissue necrosis and loss of vascular elastic fibers. The small and middle-sized arteries and veins are mainly involved and the vascular involvement frequently causes arterial aneurysms, arterial occlusions, venous occlusions and varix formations11,12).
There are different explanations about the pathogenesis of the vascular complications and the tendency for thrombosis in BD. Several investigators conduct hypercoagulable state account for clinical signs such as vascular lesions in patients with BD. Activation of platelets and coagulation system by the impairment of the intima because of the vasculitis, immunological in origin, results in hypercoagulabe conditions. Some authors suggest that increased blood viscosity and decreased prostacyclin are important factors in the formation of thrombosis13). It is claimed that prostacyclin synthesis is significantly low in patients of BD with vascular involvement. Also, some suggested that the decreased activity of the fibrinolytic system may be the probable cause of thrombosis14,15). There is a question as to whether some proteins related to coagulation are simply increased as acute phase reactants or as to whether there exists a hypercoagulable state in BD.
Pleural effusion with or without associated chest pain is usually attributed to pulmonary infarction or an infectious process and vasculitis of the pleura. In this patient, both pleural effusion and ascites may be contributed to the retrograde stasis and increased hydrostatic pressure in central large venous systems. Nonspecific findings of the pleural tissues may provide evidence that increased venous hydrostatic pressure, not pleural involvement by BD, contribute to the development of both pleural effusion.
The treatment of BD with vascular lesions remains to be solved. Treatment varies from antiaggregant agents to intravenous anticoagulation, but is usually resistant to all types of treatment. Prednisone and heparin are still used frequently in the treatment of BD. Colchicine has been recommended, chiefly because of its antichemotactic activity. Combination of these three agents is believed to be the best therapy. Some authors suggest that fibrinolytic agents could be used in patients with venous thrombosis, but aspirin treatment should not be offered due to low levels of prostacyclin in BD. The role of cytotoxic agents, such as cyclosporine-A, azathioprine and cyclophosphamide in treatment of vascular lesions of BD, is not clear.
In patients with severe collateral insufficiency, surgical intervention may be considered. The results of reconstructive vascular surgery in patients with BD are not good for venous or arterial lesions as there is a high incidence of reocclusion. So, surgical treatment should be avoided in venous lesions unless it is absolutely necessary16).
In cases of venous occlusion, the prognosis is not always poor due to the development of collateral veins and recanalitation3). But, arterial lesions have a less favorable outcome, as aneurysms may rupture, leading to death and more extensive venous lesion has limited therapy and relatively poor prognosis4). To improve the quality of life and avoid serious vascular complications, every patient with BD should be followed routinely by noninvasive vascular imaging and laboratory techniques to evaluate the arterial and venous system. Early detection and aggressive treatment of peripheral vein thrombosis are crucial for preventing propagation of the thrombus to the large vascular system.