The Imbalance between Coagulation and Fibrinolysis Is Related to the Severity of the Illness and the Prognosis in Sepsis
Article information
Abstract
Objectives
The coagulation and fibrinolytic system appears to be activated by the septic process independently, leading to the syndrome of disseminated intravascular coagulation(DIC). In this study, we investigated the changes within the hemostatic system related to the severity of the illness and the prognosis in patients with sepsis.
Methods
Plasma thrombin-antithrombin III (TAT) and plasmin-α2-antiplasmin (PAP) complexes were measured using ELISA methods in 32 patients with sepsis and 20 controls and were analyzed according to the APACHE III scores and survival of the patients.
Results
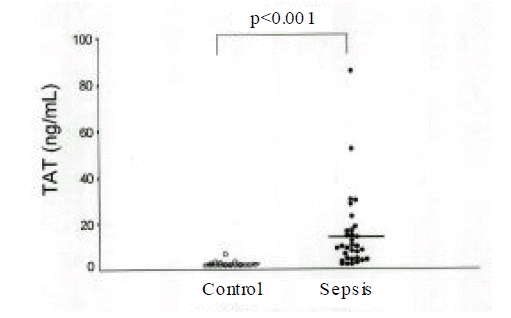
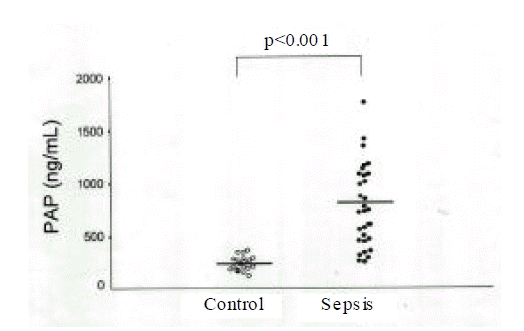
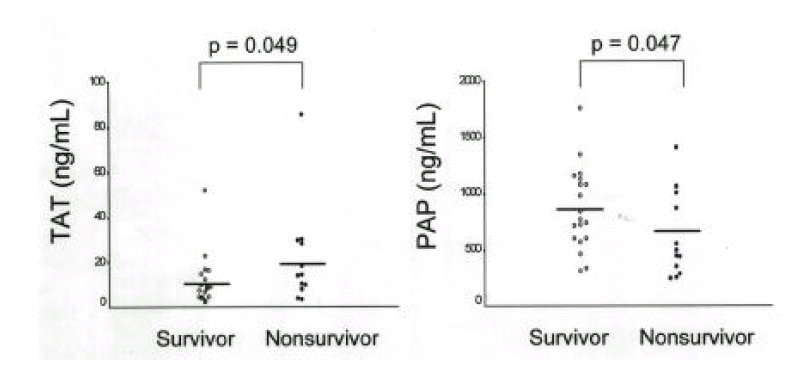
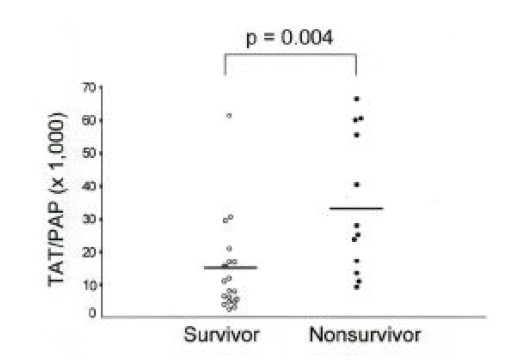
Plasma TAT and PAP in patients with sepsis were significantly higher than controls. Nonsurvivors showed greater levels of TAT(21.7±22.3ng/mL) and lower levels of PAP(628.4±378.1ng/mL) than survivors (TAT: 11.1±11.2ng/mL; PAP: 857.1±364.1ng/mL). The imbalance between coagulation and fibrinolysis described as TAT/PAP ratio was closely related with APACHE III scores in patients with sepsis(r=0.47) and the TAT/PAP ratio in nonsurvivors was significantly higher compared with survivors(34.4±21.4 vs. 14.4±13.8).
Conclusion
In sepsis, both coagulation and the fibrinolysis system are activated and the imbalance between coagulation and fibrinolysis predisposes to the hypercoagulation state and is closely related to the severity of the disease and the prognosis.
INTRODUCTION
In patients with sepsis and septic shock, the coagulation and fibrinolytic system are independently activated. Tissue factor released from endothelial cells which are stimulated by endotoxin and inflammatory mediators and the consumption of natural coagulation inhibitor antithrombin III, protein C and protein S activate the coagulation system1–3). Fibrinolysis is regulated by both activators and inhibitors released from endothelial cells4). Although the levels of plasminogen activator antigen are increased, fibrinolysis is dominated by increased levels of type 1 plasminogen activator inhibitor (PAI-1)5,6) This imbalance between coagulation and fibrinolysis predisposes to the development of disseminated intravascular coagulation, fibrin deposition and microthrombi. Fibrin deposition and complement activation can cause extensive vessel wall damage and may be associated with multiple organ failure2,3). There have been several clinical studies concerning biochemical evidence of activation of coagulation and inhibition of fibrinolysis in sepsis4,6). Thrombin-antithrombin III(TAT) and plasmin-α2-antiplasmin(PAP) complexes are known as highly sensitive and specific markers of coagulation and the fibrinolytic system7,8). Recently, it has been reported that the imbalance between coagulation and fibrinolysis described as TAT/PAP ratio leads to mortality and organ dysfunction in patients with sepsis9,10). In this study, we investigated what kind of changes within the hemostatic system are related to the severity of illness and the prognosis in patients with sepsis.
MATERIALS AND METHODS
Subjects
We prospectively studied 32 patients with sepsis (20 male, 12 female) admitted from August 1996 to July 1997. Age (mean ± SD) was 59.4 ± 17.8 years. The diagnosis of sepsis was according to the criteria described by the American College of Chest Physician/Society of Critical Care Medicine(ACCP/SCCM) Consensus Conference held in 199111). The diagnosis of sepsis was made if two of the four following criteria were fulfilled: temperature > 38°C or < 36°C, respiratory rate > 20/min or PaCO2 < 32mmHg, white blood cell count > 12,000/mm3, or < 4,000/mm3, or immature neutrophil > 10% with evidence of infection. The septic shock was defined as the sepsis-induced hypotension or vasopressor dependency and the presence of signs of hypoperfusion, such as lactic acidosis, altered mental status or oliguria.
The origins of infection were pulmonary (22), urogenital (4), wound (4), and abdominal (2) foci. Bacteremia was noted in 9 cases (4 Gram-positive, 5 Gram-negative organisms), septic shock in 8 cases and acute respiratory distress syndrome in 5 cases. 12 of 32 patients expired mainly due to multiple organ failure.
The patients with underlying cardiovascular disease, pregnancy and liver disease, as well as those on drugs affecting the hemostatic system, such as wafarin, were excluded. Control values were obtained from 20 healthy volunteers (M:5 F:15, Age: 32.4 ± 5.7 years) among the laboratory and technical personnel. The age of the control group was not matched with the patient group; this limitation may not significantly influence the result of this study because the fibrinolytic enzyme activity does not show much variation according to age, but is only slightly decreased in elderly12).
Clinical Assessment
The clinical status of the patients was assessed using the APACHE III scoring system13). APACHE III score was obtained within 24hrs after clinical determination of the sepsis. If more than 3 parameters were missing, the case was excluded. APACHE III score of the sepsis patients was 60.3±39.0(range: 9 – 176).
Determination of Plasma TAT and PAP
Blood samples were collected within 24hrs after the clinical diagnosis of sepsis. Venous blood was collected in 5-ml tubes (Vacutainer, Becton-Dickinson, Plymouth, UK) containing 0.5ml sodium citrate(0.13 mol/L). Plasma was separated by centrifugation at 1,500 × g for 15 min at 4°C within 30 min, and stored at −70°C until assay. Plasma TAT and PAP were measured by commercially available test kits according to the instructions of the manufacturers. Plasma TAT was measured by a sandwich ELISA (Enzygnost-TAT, Behringwerke AG, Marburg, FRG) which uses polyclonal rabbit antibodies against human thrombin and peroxidase-conjugated antibodies to human antithrombin III. Plasma PAP was measured by a one-step sandwich ELISA (Enzygnost-PAP, Behringwerke AG, Marburg, FRG) using monoclonal antibodies (PAP-6) against human PAP and peroxidase-conjugated antibodies to plasminogen.
Statistical Methods
Student’s t test and chi-square test were used for comparison of the mean values in the various groups. In addition, Pearson’s correlation coefficients were calculated for the different variables. All results with p values of less than 0.05 were considered as statistically significant.
RESULTS
Plasma TAT and PAP in patients with sepsis and controls
Plasma TAT and PAP in 32 patients with sepsis were 15.1±16.8ng/mL and 771.3±380.3ng/mL and the values in 20 controls were 2.8±1.1ng/mL and 240.4±69.7ng/mL (Fig. 1, 2). Although plasma TAT and PAP in patients with sepsis were significantly higher than those in controls, the TAT/PAP ratio (21.3±19.0, expressed as TAT/PAP × 1,000) in patients with sepsis was not significantly different from that (12.3±4.4) in 20 controls.
Relationship of plasma TAT and PAP to clinical features in patients with sepsis
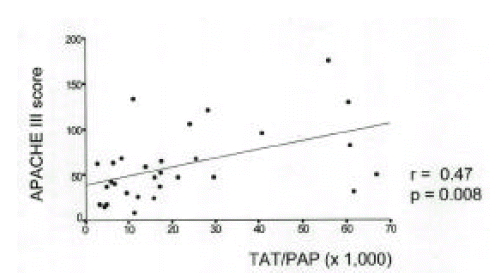
Plasma TAT and PAP in 20 survivors were 11.1±11.2ng/mL and 857.1±364.1ng/mL and those in 12 nonsurvivors were 21.7±22.3ng/mL and 628.4±378.1 ng/mL. The TAT/PAP ratio (34.4±21.4) in nonsurvivors was significantly higher than that (14.4±13.8) in survivors (Fig. 3, 4). Plasma TAT and PAP in 8 patients with shock were 11.3±9.0ng/mL and 602.1±495.0ng/mL and those in 24 patients without shock were 16.3±18.7ng/mL and 827.7±327.3ng/mL. The TAT/PAP ratio (27.2±22.5) in patients with shock was not significantly different from that (20.1±18.5) in patients without shock. No significant differences in TAT, PAP and TAT/PAP ratio were shown according to the presence of causative organisms, bacteremia, acute respiratory distress syndrome or other organ failure (data not shown). APACHE III score (93.5±41.8) in nonsurvivors was significantly higher than those (38.8±17.6) in survivors. In contrast to plasma TAT and PAP, Plasma TAT/PAP ratio in patients with sepsis was closely correlated with APACHE III score(r=0.47, p=0.008) (Fig. 5).
Relationship of plasma TAT and PAP to other hemostatic parameters
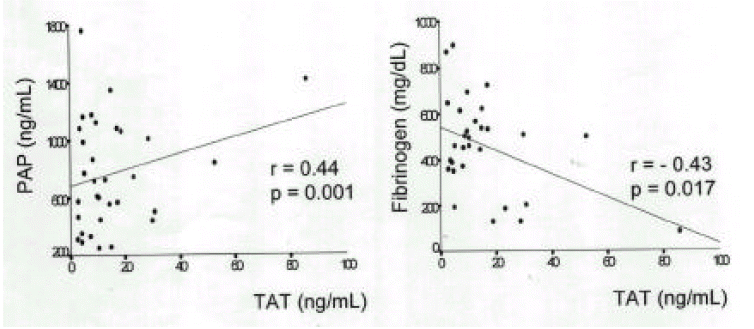
Plasma TAT was significantly correlated with plasma PAP (r=0.44, p=0.001) and negatively correlated with fibrinogen (r=−0.43, p=0.017) (Fig. 6). Dividing the patients with sepsis into two groups according to the d-dimer levels, Plasma TAT (20.2±21.7ng/mL) and PAP(1234.5±1270.0ng/mL) in patients with d-dimer level equal to or greater than 500ng/mL were significantly higher than plasma TAT (12.4±13.5ng/mL) and PAP(626.8±357.0ng/mL) in patients with d-dimer level less than 500ng/mL.
DISCUSSION
Despite the advances in antibiotic therapy and cardiopulmonary support, sepsis with septic shock remains as a highly lethal condition In sepsis, endotoxin of Gram-negative bacteria and capsular mucopolysaccharides of Gram-positive bacteria trigger the inflammatory reaction and hemostatic cascades. Endothelial cells which are stimulated by endotoxin and inflammatory mediators, express tissue factor and factor XII, resulting in activation of the coagulation pathway and consumption of the natural coagulation inhibitors, antithrombin III, protein C and protein S, also activate the coagulation system1,2,14). Platelets, aggregating to the endothelial cells damaged by protease or complement, are actively involved in the coagulation pathway4,15). Fibrinolysis is regulated by both activators and inhibitors which are released from endothelial cells. Although the levels of plasminogen activators are increased following activation of coagulation, fibrinolysis is relatively impaired compared with the activity of coagulation, and increased levels of type I plasminogen activator inhibitor (PAI-1) is suggested to play this role. This imbalance between coagulation and fibrinolysis predisposes to the development of disseminated intravascular coagulation (DIC), fibrin deposition and microthrombi. Fibrin deposition and complement activation can cause extensive vessel wall damage and may be associated with multiple organ failure6,16,17,18).
In previous reports, many hemostatic parameters, including prothrombin time (PT), partial thromboplastin time (PTT), fibrinogen, d-dimer, fibrin degradation product (FDP), plasminogen and plasminogen activator inhibitor (PAI), were studied in patients with sepsis and showed various activation patterns3,4).
Thrombin and plasmin are rapidly neutralized and stabilized by antithrombin III and α2-antiplasmin to form TAT and PAP, respectively. So, TAT and PAP are known as stable, accurate and sensitive markers of coagluation and fibrinolysis system7,8). Compared with FDP, d-dimer and antithrombin III, they have been known to be more useful parameters in the evaluation of disseminated intravascular coagulation7,8,16).
The balance of coagulation and fibrinolysis can be expressed as TAT/PAP ratio. Some variations of TAT/PAP ratio from the imbalance between coagulation and fibrinolysis have been shown according to the underlying diseases associated with disseminated intravascular coagulation. The TAT/PAP ratio was high in sepsis, intermediate in solid tumor and low in hematologic malignancy such as acute promyelocytic leukemia. High TAT/PAP ratio in patients with sepsis means excessive activation of coagulation and more vulnerability to microthrombi, microembolization and eventual multiple organ failure16,17).
In previous studies, plasma TAT, tissue-type plasminogen activator (t-PA), urokinase-type plasminogen activator (u-PA) and PAI-1 in patients with sepsis were reported to be elevated17,18). Lorente et al.9) reported that plasma TAT had a close relationship with prognosis, although plasma TAT shows no significant correlation with APACHE score or multiple organ failure score, and that higher PAI-1 contributed to poor outcome.
Recently, Kidokoro et al.10) reported that plasma TAT and PAP in patients with sepsis were elevated and high TAT/PAP ratio from the imbalance between coagulation and fibrinolysis led to the onset of organ dysfunction. Philippe et al.6) reported that plasma PAI-1 levels in patients with sepsis, causing relative impairment of fibrinolysis, were closely related with the severity and outcome.
In our study, both TAT and PAP in patients with sepsis were obviously elevated. It was shown that the high TAT/PAP ratio in patients with sepsis was closely related with APACHE III score and outcome although the relationship of TAT/PAP ratio with organ dysfunction was not clearly shown, which might be due to the small sample size. The suppression of the fibrinolytic system was more prominent in nonsurvivors than survivors.
In conclusion, both coagulation and the fibrinolysis system were activated in patients with sepsis and the imbalance between coagulation and fibrinolysis is closely related with the severity and outcome of patients with sepsis.