Imported Tertian Malaria Resistant to Primaquine
Article information
Abstract
In Plasmodium vivax and Plasmodium ovale malaria, some of the liver stage parasites remain dormant. The activation of these dormant forms (called hypnozoite) can give rise to relapse weeks, months or years after the initial infection.
To prevent relapses, a course of primaquine may be given as terminal prophylaxis to patients. Different strains of Plasmodium vivax vary in their sensitivity to primaquine and, recently, cases of relapse of Plasmodium vivax after this standard primaquine therapy were reported from various countries.
We reported a case of primaquine resistant malaria which initially was thought to be relapsed caused by loss of terminal prophylaxis.
INTRODUCTION
It has been claimed that tertian malaria had been completely eradicated in the latter part of 1970 in Korea1). But since 1993, this tertian malaria has reemerged to infect soldiers and civilians living near the armistice line2), resulting in 1,724 cases in 19973). Also, imported malaria from an overseas trip has become a medical problem to the extent of calling attention. Reports have appeared calling attention to what has been termed primaquine resistance in Plasmodium vivax in Korea4), 5).
We present a case of primaquine resistant tertian malaria which was thought to be relapsed because of loss of radical cure at initial treatment.
CASE REPORT
A 50-year-old man presented on November, 1997 with high fever, chills and splenomegaly. Three weeks before admission, he had traveled to Ethiopia where he had remained for 10 days without prophylactic chemotherapy against malaria.
The temperature was 39°C, the pulse was 100/min and the respiration rates were 22/min. The blood pressure was 110/80 mmHg.
A physical examination was normal, except for a nontender, firm splenomegaly exceeding 5cm below the costal margin. An ultrasonogram of the abdomen revealed enlargement of the spleen and slight hepatomegaly. Laboratory examinations showed hemoglobin 16.1 gm/dl, white blood cell count 7,510/mm3, platelet count 49,100/mm3, alkaline phosphatase 248 IU/L, alanine aminotransferase 165 IU/L, aspartate aminotransferase 89 IU/L. At that time, microscopic examination of a blood smear was said to show malaria (P.vivax) and a diagnosis of malaria was made. After treatment with chloroquine (750 mg as a loading dose and then 350 mg each 6, 24, 48 hours later), he felt well but subsequently did not receive primaquine because of the physician’s mistake.
After about three months, he became ill with fever and chills. Laboratory examinations showed hemoglobin 16.5 gm/dl, white blood cell count 4,150/mm3, platelet count 26,300/mm3. Microscopic examination of a stained blood smear for malarial organism (38,180/μl) was positive. The patient was given a conventional three-day course of chloroquine with relapsed malaria. On the following day, he became afebrile and reevaluation of blood smears showed decrease in the number of parasite counts(3,480/μl). He subsequently was given primaquine (15 mg base daily for 14 days) with improvement. The test of G-6PDH (glucose-6-phosphate dehydrogenase) deficiency was negatived(11.7 U/g).
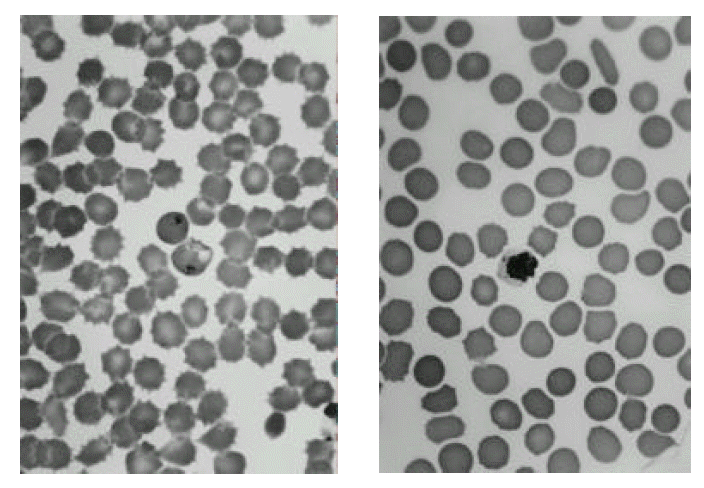
About three months after the first relapse, however, he revisited the hospital with fever, headache and chills. Physical examination showed no change, except that the spleen was felt 4cm below the costal margin. Peripheral blood examination showed variable forms of P.vivax (Fig. 1) with reduction in platelet count (97,000/mm3). He was given a diagnosis of primaquine resistant malaria. The patient was treated with chloroquine in the same dose as the in first attack. On the next day, he became afebrile and smear for malarial organisms had become negative. He subsequently received primaquine (22.5 mg daily for 21 days) without side effects from the drug. One month later, he was well and the spleen was no longer palpable. Symptoms have not relapsed during 5 months of observation.
DISCUSSION
Malaria is the world’s most important parasitic infection. Although it has been eradicated from temperate zones, increasing numbers of travelers from temperate areas each year visit tropical countries where the disease remains a major cause of morbidity and death.
Relapse occurs with P.vivax infections because delayed developmental forms of the parasite, called hypnozoites, are present in the liver. Hypnozoites can mature months to years later to cause clinical disease. After maturing and multiplying in the liver, the parasites enter a red blood cell for further development. Clinical disease is produced when parasites are released from red blood cells. A radical cure is achieved for P.vivax infections by the use of both blood-stage antimalarial drugs and primaquine, which is the only available drug that can destroy hypnozoites. To provide radical cure of P.vivax malaria, treatment must be prescribed to eliminate the liver stage of the parasites, otherwise intermittent relapses with clinical symptoms will continue.
Usually, after acute phase therapy with chloroquine, patients with P.vivax are treated with the standard course of primaquine, i.e. 15 mg base/day for 14 days, as curative therapy. Primaquine-resistant strains have been reported from Papua New Guinea, South-East Asia and Central and South America. Cases of primaquine treatment failure have also been reported in patients who visited Kenya, Sudan and Ethiopia6). The interval between primary infection and relapse varies with differing strains of P.vivax-Chesson strain from Papua New Guinea relapsing rapidly, at intervals of 28 days; strains from Southern China may relapse on an approximately annual basis. Such strains appear to show intrinsic differences in sensitivity to 8-aminoquinolines, the agents most commonly used to eradicate the liver stage. Studies carried out in the Western Pacific Region soon after World WarII, and predominantly involving the Chesson strains, indicated that a regimen of primaquine base 15 mg/day for 14 days led to failure in over 30 % of cases7). It would appear that this investigation regimen was selected solely on the basis of limiting potential toxicity. Common practice over subsequent decades within this region has been to use a regimen of primaquine base 22.5–30 mg/day for 14 days to ensure a high chance of parasite eradication8). In contrast, however, studies during the Vietnam War determined that strains from South-East Asia were more primaquine-sensitive and were almost entirely eradicated with primaquine base 15 mg/day for 14 days9).
Strains from South-East Asia and Central and South America have also been reported to relapse after standard regimens of primaquine10)–12). Most authorities agree that it is reasonable to treat patients who acquire their infections from these areas with a higher dose regimen13). To reduce side effects, a regimen of 15 mg daily of primaquine base for 28 days has been used instead of 30 mg base daily for 14 days, under the assumption that it is the total, cumulative dose of primaquine that is important in eliminating hypnozoites. Unfortunately, there are no data to support the longer duration regimen. Human and animal studies have only used total doses scheduled over a shorter time interval (60 mg base daily for 7 days versus 30 mg base daily for 14 days) in showing equivalent cure rates14–15).
It is curious that primaquine resistance emanates from areas where higher doses of primaquine have been used for longer periods. Whether this increased anti-malarial load is a risk factor for primaquine tolerance is uncertain. Animal and human studies of sub-therapeutic doses of primaquine in P.vivax infections resulted only in resistance in the erythrocytic stages of the life cycle16). While it has been suggested that the apparent increasing resistance to primaquine in P.vivax actually reflects unrecognized chloroquine-resistance in this parasite, at present, recurrence of vivax parasitemia after primaquine therapy and > 28 days from initial infection more often represents primaquine rather than chloroquine failure. Cause of the first relapse in our patient is thought to be inadequate regimen rather than chloroquine resistance: primaquine was not prescribed at initial infection. Second attack was due to primaquine resistance, since the peripheral blood smear of our patient was normal up to three days after the treatment (chloroquine) and relapse took place after three months. Moreover, he had traveled to Ethiopia where primaquine treatment failure has been reported6).
Primaquine may cause nausea and abdominal pain, particularly if taken on an empty stomach and, more important, oxidant hemolysis with methemglobinemia, anemia and, sometimes, hemoglobinuria. Patients with a deficiency of glucose-6-phosphate dehydrogenase are particularly vulnerable to oxidant hemolysis, and primaquine is contraindicated in patients with severe form of the deficiency. In places where mild variants of glucose-6-phosphate dehydrogenase deficiency are common, primaquine (0.8 mg of base per kilogram; adult dose, 45 mg) should be given once a week for six weeks for a radical cure17).
It is imperative to establish the most appropriate regimen with primaquine for the curative treatment of relapsed vivax malaria after the completion of chloroquine-primaquine therapy.