Eosinophil Activation Markers in Induced Sputum in Asthmatics
Article information
Abstract
Objectives
Eosinophils play an important role in asthmatic airway inflammation collaborately with other inflammatory cells. The present study was aimed to determine whether the eosinophil activation markers in induced sputum reflect the clinical status in asthmatics.
Methods
The clinical severity and FEV1 were measured. Hypertonic saline-induced sputum was obtained from 25 asthmatics and ten control subjects. We processed freshly expectorated sputum separated from saliva by treatment with an equal volume of dithiothreitol 0.1%, cytospins for cell count and special stain, and a collection of the supernatant for biochemical assay. We used a fluoroimmunoassay to detect eosinophil cationic protein (ECP), and a sandwich ELISA to detect interleukin (IL)-5.
Results
Asthmatics, compared with control subjects, had a significantly higher proportion of eosinophils (25.6 ± 4.6% vs 1.7 ± 0.2%, p <0.01) and higher levels of ECP (1117.8 ± 213.9μg/L vs 154.6 ± 47.4μg/L, p<0.01) in their sputum. IL-5 was detected more frequently in asthmatics than in control subjects [11/25 (44%) vs 1/10 (10%), p <0.05]. Moderate to severe asthmatics had a significantly higher proportion of eosinophils, higher levels of ECP and IL-5 compared to mild asthmatics.
FEV1, FEV1/FVC were significantly correlated with the proportion of eosinophils and the levels of ECP and IL-5. Significant positive correlations were noted between the proportion of eosinophils and the level of ECP and IL-5. Sputum ECP level showed a significant positive correlation with IL-5 level.
Conclusion
These findings demonstrate that eosinophils and the eosinophil activation markers, such as ECP and IL-5 in induced sputum, are closely related to the clinical status in asthmatics. Induced sputum study may thus be useful in clinically measuring indices of airway inflammation in asthma.
INTRODUCTION
Bronchial asthma has been characterized by infiltration of eosinophils into bronchial wall and respiratory epithelial damage. These changes consist of four steps, including enhanced eosinophil production, recruitment into lung tissue, activation of eosinophils and release of inflammatory mediators. Degranulation of eosinophilic granular proteins, such as eosinophil cationic protein (ECP), major basic protein (MBP), eosinophil-derived neurotoxin (EDN) and eosinophil peroxidase (EPX), is fundamental to the proinflammatory role of eosinophils. These granular proteins damage the airway epithelium, stimulate the release of histamine from mast cells, contract the airway smooth muscle and promote airway hyper-responsiveness1,2).
Recent studies have documented that several cytokines, such as interleukin (IL)-5, granulocyte-macrophage colony stimulating factor (GM-CSF), IL-3, tumor necrosis factor (TNF)-α, interferon (INF)-α, IFN-β, IFN-γ, IL-1, IL-4, platelet activating factor (PAF), regulation-upon-activation, normal T expressed and secreted (RANTES) and macrophage inflammatory protein (MIP)-1α, activate eosinophils3). Especially, IL-5, a cytokine that attracts, activates and prolongs the survival of eosinophils, is most important in the pathogenesis of allergic inflammation and asthma, and it contributes to eosinophil viability in the sputum of asthmatics during attacks4).
Collectively, ECP and IL-5 may be increased in asthmatic airway inflammation with activated eosinophils and they can be used as the activation markers of eosinophils.
The airway inflammation in bronchial asthma has been studied directly by bronchoscopic biopsies5–7), bronchoalveolar lavages (BAL)8–10) or sputum examinations11,12), and indirectly by measurements in peripheral blood13,14). Recently, an examination of sputum, induced by the inhalation of hypertonic saline has introduced and is known to be a valuable and reproducible method to evaluate the eosinophilic inflammation in asthma.
Therefore, this study was designed to determine whether eosinophils and the eosinophil activation markers in the sputum obtained using this new method reflect the clinical status in asthmatics.
METHODS
1. Subjects
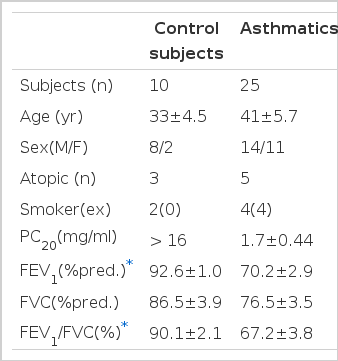
Twenty five patients with asthma and ten control subjects were recruited for this study from the Division of Allergy, Department of Internal Medicine, Chonnam University Hospital between March and September, 1997 (Table 1). Control subjects, who volunteered for this study, had no history of any respiratory symptoms, and had forced expiratory volume in one second (FEV1)>75% predicted, a ratio of FEV1 to forced vital capacity>75% and a normal methacholine airway responsiveness [provocation concentration of methacholine producing a 20% fall of FEV1 (PC20)>16mg; AHR]. The diagnoses of asthma were established in the patients by their symptoms of recurrent episodic wheezing, cough and/or dyspnea, accompanied either by methacholine airway hyper-responsiveness (thirteen patients) or by a significant improvement of FEV1 (15%) following antiasthma therapy (five patients). No subject had respiratory infection for 4 weeks prior to the study. The study was approved by Chonnam University Hospital Research Committee and all subjects signed the informed consent forms.
2. Study design
Eosinophils and eosinophil activation markers in hypertonic saline-induced sputum were measured in asthmatic patients and compared with those in control subjects. On the first visit, a questionnaire for symptoms and medications was given and a spirometry was performed. After that, induced sputum was collected and skin prick tests and methacholine provocation tests were done. Clinical severity of asthma was classified by the method of the International Consensus Report15). All measurements in sputum were obtained by a person blind to the clinical characteristics of subjects.
Spirometry was performed according to the American Thoracic Society standards16) using SensorMedics 2200 spirometer (Cardiopulmonary Care Company™, Yorba Linda, California). The representative values for FVC and FEV1 were selected according to the International Thoracic Society criteria17) and the reference values were taken from the reports by Choi et al.18) and by Kim et al.19). Methacholine challenge tests were carried out by a modification of the method described by Chai et al.20) and the results were expressed as PC20 in noncumulative units. Allergy skin prick tests were performed with 55 common allergen extracts. Atopy means one or more positive allergy skin prick tests.
3. Sputum induction
Sputum induction was performed as a modification of the method described by Fahy et al.21). All subjects were premedicated with inhaled salbutamol 2 puffs (200μg). Subjects inhaled 3% hypertonic saline solution aerosols generated by an ultrasonic nebulizer (NE-U03, OMRON Co., Tokyo, Japan) with maximum output of 0.15–0.3 ml/min and mass median aerodynamic diameter of 4.5μm Hypertonic saline was inhaled for 20–30 min according to the severity of asthma until an adequate volume of sputum was expectorated. Subjects were asked to rinse the mouth and blow the nose to minimize contamination with saliva and postnasal drip. They were encouraged to cough deeply and frequently during hypertonic saline inhalation. They were instructed to cough the sputum into a sterile plastic container. The volumes of samples and duration of sputum induction were recorded. FEV1 was measured before, during and after induction of sputum. Sputum induction was stopped in subjects with a fall of the FEV1 ≥15%.
4. Sputum processing
The appearance of the sputum was recorded as mucoid, mucopurulent or purulent. Sputum was selected from saliva and processed within 2 hours. The method of sputum examination described by Popov et al.22) was modified21). Sputum was treated by adding equal volumes of 0.1% dithiothreitol (Sputalysin 10%; Gibco BRL, USA) followed by equal volumes of Dulbecco’s phosphate buffered saline (D-PBS). The sample was then mixed gently and placed in a shaking water bath at 37°C for 15 min to ensure complete homogenization. The sample was removed from the water bath periodically for further brief gentle mixing. The suspension was filtered through a gauze (1mm pore size), the filtrate was centrifuged at 1,500 rpm for 10 min and the supernatant was aspirated and stored in Eppendorf tubes at −70°C for later assay. The cell pellet was resuspended in D-PBS, 1000μL and total nonsquamous cells were counted in a modified Neubauer hemocytometer. The cell suspension was adjusted to 0.5×105/ml, and then 50 μL of cell suspension placed into cups of Sakura cytocentrifuge (Model CF-127, Tokyo, Japan), and two coded cytospins were prepared at 600 rpm at 5 min, air dried, stained by Diff-Quick® (Kookje Scientific Products, Japan) stain and stored at −70°C. Cell differentials of 400 nonsquamous cells were performed in Diff-Quick® stain slides by two investigators who did not know the subject’s history, and the results were expressed as a percentage of the total non-squamous cell count.
5. ECP and IL-5 measurement
The concentration of ECP in 400μL in the thawed supernatant was determined using fluoroimmunoassay (Pharmacia CAP system). IL-5 was measured by quantitative sandwich enzyme immunoassay (Quantikine™; R&D Systems, Inc., MN), as described by Dickason23). Samples were analysed in duplicates. The limits of detection for ECP and IL-5 assays were 2.0 μg/L and 3 pg/ml, respectively.
6. Statistical analysis
All data were analyzed using the SPSS version 7.5 for Windows. Data are expressed as mean ± SEM. Comparison of variables was performed using Student’s t test and Mann-Whitney U test. One-way analysis of variance (ANOVA) was used to compare mean values among groups of subjects. When a significant difference between groups was observed, inter-group comparisons were assessed by Bonferroni’s method. Pearson’s correlations and Spearman’s correlations were used to assess relationships between variables. A p-value of <0.05 was considered significant.
RESULTS
Subject characteristics are given in Table 1. Asthmatics had significantly lower FEV1, FEV1/FVC than control subjects.
1. Safety of sputum induction
Sputum induction by hypertonic saline inhalations was discontinued if the FEV1 fell by more than 15%. The subjects experienced a salty sense in thirtyeight sputum induction trials, chest tightness in three and dyspnea in two. Mean duration of the induction was 15.6 min in asthmatics and 19.6 min in control subjects. During sputum induction, there were falls of FEV1 ≥ 15% in two asthmatics which required additional inhalations of salbutamol aerosols. The maximal fall in FEV1 from baseline during the hypertonic saline inhalation was significantly greater in the asthmatics than in the control subjects (5.2 ± 0.6% vs 1.5 ± 0.9%, p<0.01).
2. Differential counts of sputum cells
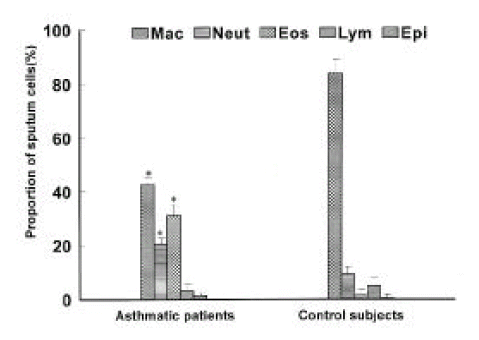
Thirty eight sputum samples from asthmatics and 10 sputum samples from control subjects were analyzed. The sputum had less than 30% of squamous epithelial cells and greater than 85% of cell viability. Differential counts in the induced sputum are shown in Figure 1. The proportion of eosinophils and neutrophils was significantly higher in asthmatics than in control subjects (eosinophils: 25.6 ± 4.6% vs 1.7 ± 0.2%, p<0.01; neutrophils: 20.3 ± 2.4% vs 9.4 ± 1.8%, p<0.01), whereas the proportion of macrophage was lower (42.8 ± 5.3% vs 84.0 ± 1.6%, p<0.01). No significant differences were noted between asthmatics and control subjects in either total cell count (7.4 ± 2.2 × 105/ml vs 5.3 ± 0.9 × 105/ml) or the absolute or relative numbers of lymphocytes and epithelial cells.
3. Eosinophil activation markers
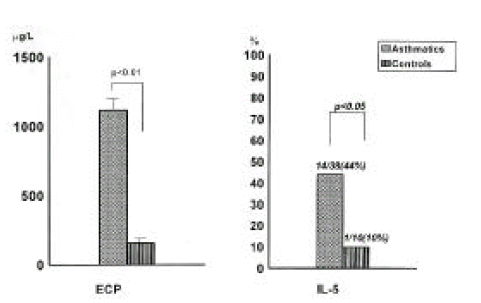
The level of ECP in the sputum was significantly increased in asthmatics compared with that in control subjects (1117.8±213.9μg/L vs 154.6 ± 47.4μg/L, p<0.01, Figure 2). IL-5 was detected more frequently in asthmatics than in control subjects [11/25 (44%) vs 1/10 (10%), p<0.05].
4. Sputum eosinophils and eosinophil activation markers by asthma severity
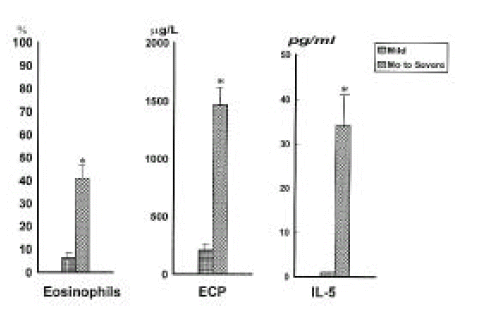
The proportions of eosinophils and ECP level in the sputum were significantly higher in moderate to severe asthma (n=18) than in mild asthma (n=7) (p<0.01, respectively, Figure 3). IL-5 was significantly increased in moderate to severe asthmatics compared to mild asthmatics (34.2 ± 8.5pg/ml vs 0pg/ml).
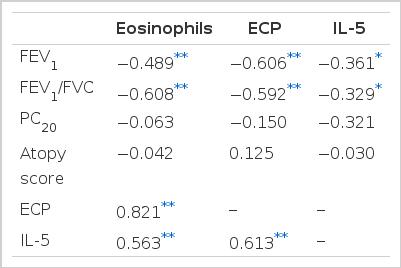
5. Correlations between eosinophil activation markers and clinical findings
The proportion of eosinophils in induced sputum was inversely correlated with FEV1 and FEV1/FVC (Table 2). The eosinophil activation markers (ECP and IL-5) had also an inverse relationship with FEV1 and FEV1/FVC. Eosinophil activation markers did not correlate atopy score, methacholine-AHR.
6. Correlations between sputum eosinophils and eosinophil activation markers
The proportions of sputum eosinophils were positively correlated with the ECP level and the IL-5 level (Table 2). The level of ECP was positively correlated with the level of IL-5.
Discussion
Asthma is characterized by reversible airway obstruction and chronic airway inflammation in which eosinophils are known to play a major role. Until recently, direct measurement of indices of airway inflammation was only possible by postmortem examination1–2), bronchoscopic biopsy5–7,29) or BAL8–10). These invasive procedures are not suitable to monitor inflammation in clinical practice. A non-invasive method has been developed using sputum induced by the inhalation of hypertonic saline to quantify and characterize inflammatory cells27). The examination of sputum selected from saliva, use of dithiothreitol to disperse the mucus and use of cytospins provide a direct research tool to measure activation markers, inflammatory mediators and cellular functions pertinent to asthma pathogenesis non-invasively24). Recently, de la Fuente PT et al. reported that hypertonic saline-induced sputum is a safe technique even in patients with severe asthma25). Sputum induction is not only non-invasive and easily repeated but also yields samples more concentrated and richer in airway secretions than those obtained by bronchoscopy28).
The present study using this new non-invasive method showed that asthmatics, compared with control subjects, had significantly higher proportions of eosinophils and higher levels of ECP in the induced sputum. IL-5 was detected more frequently in asthmatics than in control subjects.
An increase in eosinophils in the sputum of asthmatics was observed more than 100 years ago24). Several investigators11,24,31) have reported higer proportion of eosinophils and high levels of ECP in sputum in asthmatics. In accordance with a previous study, we found that asthmatics had a higher proportion of eosinophils and higher levels of ECP. Sputum ECP levels were more closely correlated to lung function parameters than serum ECP concentrations and/or microscopic sputum analysis31). Moderate to severe asthmatics showed significantly higher levels of eosinophil activation markers than mild patients. FEV1 and FEV1/FVC were significantly related to these markers11,24). An increase in inflammatory cells associated with increased expression of activation markers for lymphocytes and for eosinophil secretion is found even in stable asthma26). There was inverse correlation between eosinophils in induced sputum and bronchial responsiveness32). In the present study, the proportions of eosinophils and eosinophil activation markers were negatively correlated with severity of airway obstruction (FEV1, FEV1/FVC). We did not find any correlation between eosinophil activation markers and methacholine-AHR, which may be derived from the difference in patients select in this study.
An increased level of IL-5 in induced sputum in asthmatics has been demonstrated11,30). Adachi et al4) showed that eosinophil viability-enhancing activity present in sputum from exacerbated asthmatics was related to IL-5 and GM-CSF. In the present study, asthmatics with moderate to severe disease had higher levels of IL-5 than those with mild severity. IL-5 was significantly, positively correlated with the proportion of eosinophils and the levels of ECP, and negatively correlated with FEV11,30). In the present study, the concentration of IL-5 was correlated with the proportions of eosinophils and the levels of ECP. These results prove that IL-5 is one of the cytokines that influence the activation of eosinophils in asthmatics. The correlation between the proportion of eosinophils and IL-5 suggests that eosinophils may be highly activated by IL-5. Further studies are needed to clarify the relation between IL-5 and other cytokines, such as GM-CSF and IL-3, and chemokine, such as RANTES and eotaxin.
In summary, we have found higher proportions of eosinophils and high concentrations of eosinophil activation markers in induced sputum with asthmatics. We conclude that eosinophil activation markers in the tracheobronchial secretion are closely associated with the severity of asthma and that the examination of induced sputum can be used to non-invasively evaluate airway inflammation in asthma.