Increased Prevalence of Autoimmune Thyroid Disease in Patients with Type 1 Diabetes
Article information
Abstract
Background
Type 1 diabetes mellitus is frequently associated with other autoimmune diseases. The occurrence of common features of autoimmune diseases and the coassociation of multiple autoimmune diseases in the same individual or family supports the notion that there may be common genetic factors.
Methods
To investigate potential clustering of autoimmune thyroid disease (ATD) among type 1 diabetes patients and the contribution of common susceptibility genes to this, HLA DR/DQ alleles as well as antithyroid autoantibodies were measured in 115 Korean patients with type 1 diabetes and their 96 first-degree family members.
Results
Twenty-five percent of the patients had ATD, whereas 3 of 36 (8%) age-matched normal controls had ATD (RR = 3.7, p < 0.05). Twenty-six of ninty-six (27%) type 1 diabetes family members had ATD. No differences in the distribution of HLA alleles/haplotypes and genotypes between the patients with and without ATD were found.
Conclusion
From this finding, we could assess that individuals with type 1 diabetes and their relatives frequently develop ATD, perhaps due to common susceptibility genes that are shared among first degree relatives.
INTRODUCTION
Type 1 diabetes is a disease generally occurring in children and is characterized by an absolute deficiency of insulin caused by destruction of the pancreatic cells through an autoimmune mechanism1). Genetic backgrounds are important in the pathogenesis of type 1 diabetes and, like many autoimmune diseases, type 1 diabetes is associated with particular HLA alleles2–4). During the long prodromal period, three major specificities of diabetes-associated autoantibodies to pancreatic cell antigens may arise and persist even after the disease has become clinically overt: is let cell antibodies (ICA), which stain cytoplasmic islet cell components by indirect immunofluorescence on frozen sections of human pancreas5); autoantibodies to 64kD islet cell protein from islet cell extracts, which has been reported to be glutamic acid decarboxylase (GAD)6, 7); and autoantibodies to 37/40kD islet cell protein, which has been reported to be an islet tyrosine phosphatase related molecule termed ICA512/IA-28–10). Type 1 diabetes is frequently associated with other organspecific autoimmune diseases, including autoimmune thyroid diseases (ATD), pernicious anemia and idiopathic Addison’s disease11). Moreover, type 1 diabetes patients and their relatives, even without clinical manifestations of other organ-specific autoimmune disease, have a high prevalence of thyroid microsomal, gastric parietal and adrenal antibodies that support an autoimmune etiology for type 1 diabetes12). In Korean type 1 diabetes patients, the most common coexisting organ-specific autoimmune disease is ATD. There have also been reports indicating that ATD patients have a high prevalence of autoantibodies specific for type 1 diabetes13, 14). However, we do not understand yet why they associate frequently. In addition, although type 1 diabetes patients with ATD are often reported to have increased levels of autoantibodies specific for type 1 diabetes compared with those without ATD15, 16), there has been no plausible explanation for the phenomenon. In this study, we measured ICA, anti-GAD and ICA512/IA-2 autoanbodies in type 1 diabetes patients, onset age below 15 (with and without ATD) and their family members to investigate potential clustering of ATD in type 1 diabetes patients, and to study the increase in the level of autoantibodies specific for type 1 diabetes in patients with ATD. We also wanted to investigate whether type 1 diabetes susceptible HLA DR/DQ alleles are shared by the type 1 diabetes patients with ATD to explain their coassociation.
MATERIALS AND METHODS
1. Patients
For this investigation, 115 cases of type 1 diabetes patients were selected randomly from the Korean Seoul Registry (incidence = 0.6/100,000/yr)17). All type 1 diabetes patients were unrelated and on insulin therapy at hospital discharge, below 15 in age and residents of Seoul at the time of onset of the disease. The criteria for classification of type 1 diabetes were determined by the ADA Commission criteria18). Of the 115 patients, fifty-seven were male and fifty-eight were female; their mean current age was 12 years (range: 3 – 22 years). Their mean age at diagnosis was 7.8±4.1 yrs with the mean diabetes duration of 4.6±3.4 yrs. Thirty-six non-diabetic control subjects, with no family history of diabetes, matched by sex and age, were selected from the same geographical area. Eighteen were male and eighteen were female. Their mean current age was 13.4 years (range: 1.4 – 20 years). All the patients and control subjects gave their informed consent to the study.
The presence of ATD was assessed by high titers (> 0.3 ng/mL) of thyroid peroxidase or thyroglobulin antibodies, the level of TSH and a positive medical history and/or physical exam. Details of the clinical characteristics of the type 1 diabetes patients with and without ATD are given in Table 1. Of the twenty-nine type 1 diabetes patients with ATD, two had been hyperthyroid both clinically and biochemically and treated with antithyroid drugs and maintained euthyroid during the exam. All patients were in a euthyroid state at the time of sampling as a result of receiving replacement therapy.

Clinical characteristics of type 1 diabetes patients with and without ATD (Auto immune Thyroid Disease)
A total of ninety-six family members from the forty-two simplex type 1 diabetes family also participated in this study. Forty-six were male and fifty were female. Their mean current age was 45 years (range: 38 – 64 yrs). All the genotypes of the patients with or without ATD were precisely assessed by the family study.
2. Laboratory methods
(1) Anti-thyroid autoantibodies measurement
Thyroid peroxidase and thyroglobulin antibodies were measured by radioimmunoassay using commercial kits developed by RSR Limited (UK). Thyroglobulin and thyroid peroxidase were purified from human thyroid tissue which was obtained from patients with Graves’ disease during surgical operation. After diluting serum samples 20-fold in serum diluent, 50μL aliquots of the diluted sera were incubated with 50μL of 125I-labeled thyroglobulin or thyroid peroxidase for 60 min at room temperature. Solid-phase Protein A (50μL) was then added and incubated for 60 min at room temperature. We then added 1 ml of assay diluent, centrifuged the tubes and counted the radioactivity in the pellets. The titer was expressed in “U mL-1”. A value of more than 0.3 U mL−1 was considered positive, which was 3 SD above the mean of the fifty healthy individuals.
(2) Anti-GAD immunoprecipitation assay
Soluble porcine GAD was purified from fresh pig brain and prepared for the GAD enzyme assay as previously described19). The purified and enzymatically active GAD showed both isoforms (67,000 and 65,000 MW) by sodium dodecyl sulphate-polyacrylamide gel electrophores is (SDS- PAGE). For immunoprecipitation, after preadsorption with pooled normal sera, 40μL of 125I-GAD (50,000 cpm) was added to 25μL of test plasma diluted 1:2 in cold wash buffer (20 mmol/L Tris pH 7.4, 150 mmol/L NaCl, Triton X-100 0.5% weight/volume). Samples were incubated overnight at 4°C, further incubated with 50μL of 50% protein A-Sepharose (1 hr, 4°C), then centrifuged. The precipitate was washed three times in 750μL of wash buffer and radioactivity counted. A positive standard serum, defined to contain 100 units of antibody, was included in every assay. The activity of test sera was expressed as a percentage of the counts precipitated by the reference serum. The upper limit for a normal result, as determined using sera from blood donors, is 18 U, which exceeds the mean + 3S.D.
(3) Islet cell cytoplasmic antibody (ICA) test
ICA was assessed by immunoenzymatic method on cryostat sections of pancreas from a blood group O donor obtained during a surgical operation as previously described20). The titers of ICA were expressed in Juvenile Diabetes Foundation (JDF) units and a value of more than 2.5 JDF units was considered positive. Our ICA assay was done in the University of Nagasaki and they participated in ICA proficiency tests sponsored by the International Diabetes Workshop, and our validity, consistency, sensitivity and specificity in the 5th ICA proficiency test were found to be 95%, 95%, 85% and 100%, respectively.
(4) ICA512/ IA- 2 autoantibody radioassay
Amplified cDNA corresponding to nucleotides 839-3083 of IA-2 sequence (ICA512/IA-2 (256-979)) subcloned into a pCRII vector (Invitrogen, San Diego, CA) was kindly provided by George Eisenbarth. The cDNA was transcribed and translated in vitro in the presence of 35S-methionine (Amersham International, Amersham, Bucks, UK; > 1000 Ci/mmol) using the TNTcoupled rabbit reticulocyte system (Promega). The ICA512/IA-2 radioassay was performed using a 96-well plate format similar to a recombinant GAD65 radioassay as described previously21) with some modifications. In brief, in vitro translated 35S-labeled protein (20,000 cpm of TCA precipitable protein) was incubated with patients’ serum at a 1:25 dilution overnight at 4°C in Tris-buffered saline/Tween 20 (TBST; 20 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 0.1% bovine serum albumin, 0.15% Tween 20) containing 0.1% aprotinin and 10 mmol/L benzamidine. Twenty-five (L of 50% Protein A-sepharose (Pharmacia, Uppsala, Sweden) in TBST was added to the reaction in a MultiScreen-DV 96-well filtration plate (Millipore, Burlington, MA) and incubated for 45 min at 4°C, and washed nine times with cold TBST using the Millipore vacuum-operated 96-well plate washer (Millipore). After washing, 40 μL of scintillation liquid (Microscint-20; Packard, Meriden, CT) was added to each well and radioactivity was determined directly in the 96-well plate with Top Count 96-well plate β counter (Packard). For autoantibodies to ICA512/IA-2, the positive and negative control sera were included in every assay, and the antibody levels were expressed as an index defined as: (cpm in the unknown sample – negative control)/(positive control – negative control). “Positive” for these assays were based on the 100th percentile of sera from 208 healthy control subjects, and were an index of 0.071. The inter-assay coefficient of variation (CV) and intra-assay CV were 11.7% (n=9) and 10.0% (n=10).
(5) HLA- DRB1, - DQA1 and - DQB1 gene analysis
Peripheral blood lymphocytes from all donors were used for the molecular typing of the generic typing of HLA-DRB1 and most common HLA-DQ A and B chain gene allelic forms22). To this aim, DNA was extracted from peripheral blood lymphocytes using standard techniques, and used as a template for PCR amplification23). HLA DRB1/DQA1/DQB1 was genotyped using PCR-SSO techniques according to the previous reports19, 24). Successful PCR amplifications were obtained from each individual’s genomic DNA using the thermostable Taq polymerase and a thermocycler from Perkin Elmer Cetus (Norwalk, CT, USA). The procedure was carried out using the following primers for amplification: DRB- amp A 5′- CCC CAC AGC ACG TTT CTTG-3′, DRB- amp B 5′-CCG CTG CAC TGT GAA GCT CT-3′, DQA- ampA 5′-ATG GTG TAA ACT TGT ACC AGT-3′, DQA- ampB 5′- TTG GTA GCA GCG GTA GAG TTG-3′, DQB-ampA 5′-CAT GTG CTA CTT CAC CAA CGG-3′, DQBampB 5′-CTG GTA GTT GTG TCT GCA CAC-3′. Thirty cycles of amplification were performed: denaturation at 94°C for 30 sec, annealing at 55°C for 30 sec, and 30 sec extension at 72°C. One microliter of amplified DNA was dot spotted on nylon membrane filters, denatured with 0.4N NaOH for 20 min, and then neutralized with 2x SSPE (1.5 mol/L NaCl, 0.1 mol/L NaH2 PO4, 10 mmol/L EDTA, pH 7.4). After the filters were dried at room temperature, the DNA was fixed to the filters by exposure to UV light for 5 min. The filters were prehybridized at 54°C for 2 hr in a solution of 3 mol/L tetramethylammonium chloride, 50 mmol/L Tris (pH 8.0), 2 mmol/L EDTA (pH 8.0), 0.1% SDS, 5x Denhardt’s solution (5 g Ficoll, 5 g polyvinylpyrolidone, 5 g BSA, 500 mL H2O diluted 10-fold into prehybridization buffer), and Herring’s sperm DNA (100 mg/L). The filters were hybridized at 54°C overnight in a prehybridization solution with 32P labeled oligonucleotide probes. After hybridization, filters were washed twice nonstringently for 10 minutes in 2× SSPE−0.1% SDS at room temperature. One stringent wash was subsequently performed for 10 min at room temperature and one wash at 58°C in a solution of 3 mol/L tetramethylammonium chloride, 50 mmol/L Tris (pH 8.0), 2 mmol/L EDTA (pH 8.0), and 0.1% SDS. The filters were exposed to X-ray film (Kodak XAR-5, Eastman Kodak, Rochester, NY) with an intensifying screen at −80°C for 1–4 hr. The nomenclature used to define the HLA-DR and-DQ alleles was according to the official Nomenclature for Factors of the HLA System, 199125).
3. Statistical analysis
All data are presented in the form of mean±S.D. Comparisons between the groups were made by unpaired parametric tests (students’ t-tests) and nonparametric tests (Mann-Whitney U tests). The degree of correlation between antibody levels was tested by linear regression analysis. The gene frequencies were obtained by counting the total number of specific HLA antigen defined by oligonucleotide probe analysis. Difference in the HLA allele distributions was determined using the χ2 test with Yates’ correction (two-tailed). When the expected frequency for one of the alleles was less than 5, the Fisher’s exact test was used. Corrected p values (pc) were obtained by multiplying the p value by the number of alleles tested for each locus. Estimates of the relative risk (RR) in the different individuals were calculated using Woolf’s method, as modified by Haldane for small sample sizes when appropriate26).
RESULTS
As shown in Table 1, twenty-nine of 115 (25.2%) type 1 diabetes patients had ATD, whereas 3 of 36 (8.3%) age and sex-matched normal controls had ATD (RR = 3.7, p < 0.05). Twenty-six of 96 (26.9%) type 1 diabetes family members had ATD. The type 1 diabetes patients with ATD had longer duration of type 1 diabetes and were mainly composed of females. There was no difference in the prevalence of diabetic ketoacidosis at the onset of diabetes and no difference in the frequency of family history. Neither could we find the difference of fasting c-peptide levels between the two groups (Table 1).
There was no difference in either the titer or the prevalence of ICA and anti-GAD between the patients with and without ATD (Table 2). In contrast, type 1 diabetes patients with ATD had decreased levels of ICA512/IA-2 autoantibody (0.11 (0.24 vs. 0.26 (0.45, p < 0.05). However, when we analyzed the anti-GAD titer according to duration of diabetes, we could find a significant difference. In longstanding type 1 diabetes patients with ATD (>/= 3.6 year), the prevalence of anti-GAD was higher than in those without ATD (68.4 % vs. 40.5%, p < 0.05), but the titer was not significantly different between the two groups. In contrast, in patients with short duration diabetes (< 3.6 years), the prevalence and levels of anti-GAD were not different from those in patients without ATD.
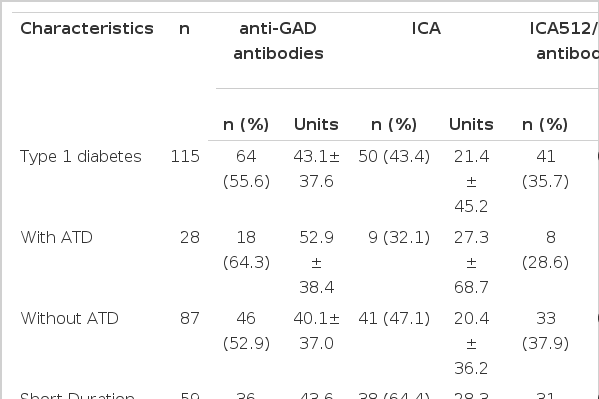
Prevalence and levels of antibodies to GAD, ICA and ICA512/IA-2 antibodies in Korean type 1 diabetes patients with and without ATD
Lower titer of ICA512/IA-2 antibody, but with similar prevalence of ICA512/IA-2 antibody positivity, was found in patients with ATD compared with the patients without ATD (Table 2). However, when we compared the ICA512/IA-2 antibody prevalence controlling for the duration, we could not find any significant difference between the two groups.
When we confined the type 1 diabetes patients to those with short duration of diabetes (< 3.6 years), the prevalence of ICA in patients with ATD was lower than in those without ATD. The prevalence and levels of ICA were 3 of 9 (33.3 %) and 33.7±57.7 U, respectively, in patients with ATD and 35 of 50 (70.0 %) and 27.4±39.2 U, respectively, in patients without ATD. In contrast, in patients with long-standing diabetes (>/= 3.6 years), the prevalence and levels of ICA were similar between the type 1 diabetes patients with and without ATD.
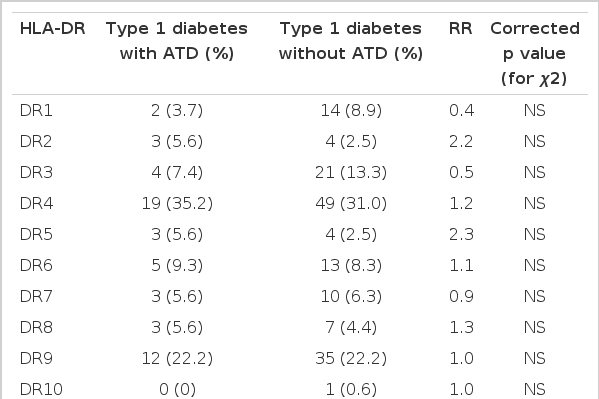
We also compared the distribution patterns of HLA-DR/DQ polymorphisms by molecular methods between the type 1 diabetes patients with and without ATD. All of the HLA-DR alleles identified in the type 1 diabetes patients with and without ATD are given in Table 3. All of the HLA-DQ alleles identified in the type 1 diabetes patients with and without ATD are given in Table 4. No differences of the distribution of HLA alleles (or type 1 diabetes susceptibility alleles) and genotypes between the patients with and without ATD were found.
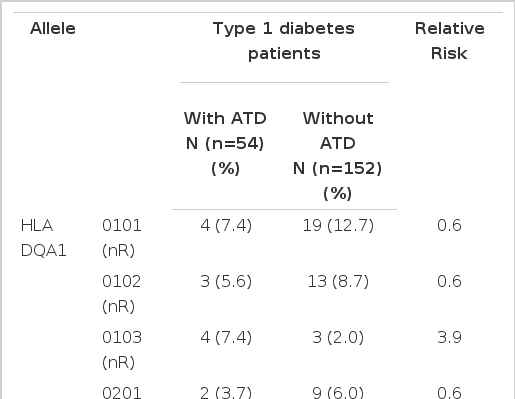
Distribution of HLA-DQA1 and DQB1 gene frequencies in type 1 diabetes patients with and without autoimmune thyroid disease (ATD)
We also measured HLA-DR/DQ polymorphisms in the first-degree family members of type 1 diabetes probands. To date, 42 type 1 diabetes probands, 40 siblings and 56 parents have participated. Six parent-offspring ATD families have been identified; 3 with ATD mothers, 2 with ATD fathers and 1 with both parents affected. Among the offspring with ATD, 75% were female (1 type 1 diabetes, 29 non-diabetic) and 25% were male (10 non-diabetics). Three parental HLA DRB1/DQA1/DQB1 haplotypes have been defined in 6 of these families; 4 with DR4/DQA1*0301/DQB1*0302, 1 with DR3/DQA1*0501/DQB1* 0201 and 1 with DR9/DQA1*0301/DQB1*0303.
DISCUSSION
Type 1 diabetes is an organ-specific autoimmune disease that culminates in the destruction of pancreatic islet β cells and insulin deficiency1). Type 1 diabetes is also associated with other organ-specific autoimmune diseases, including autoimmune thyroid disease (ATD)11, 12, 15, 16). In Korean type 1 diabetes patients, as well as in Caucasians, ATD is the most common coexisting organ-specific autoimmune disease. Individuals with type 1 diabetes patients even without clinical manifestations of other organ-specific diseases such as ATD, are reported to have a high freuency of thyroid autoantibodies12, 27, 28). However, these studies showed a wide range of the prevalence of ATD. From our study, we found a 25% prevalence of ATD in type 1 diabetes patients even in the young age, compared with 8% in age, sex matched controls. It may be feasible that more frequent screening of TFT may facilitate the identification of the individuals who are at risk for development of ATD.
As shown in Table 1, the duration of type 1 diabetes since onset in patients with ATD was significantly longer than that in type 1 diabetes patients without ATD, with no difference of age at onset. This finding is not in consistence with that of Kawasaki et al.15) in that they found a difference of age at onset rather than duration. Furthermore, they insisted that the prevalence of coma or ketoacidosis at the onset of type 1 diabetes in patients with ATD was significantly lower than that in patients without ATD, and that type 1 diabetes patients with ATD were clinically characterized by a late and slow onset of diabetes. But having no difference of basal insulin (c-peptide) secretory capacity might be an unlikely finding to explain type 1 diabetes patients with ATD as such. Neither could we confirm these findings. These differences might be resulted from the numbers and the patient’s age at onset.
There have been some reports indicating that high levels of anti-GAD antibodies were present in type 1 diabetes patients with ATD, compared with those without ATD15, 16). In those studies, autoantibodies to GAD were determined by radioimmunoassay using the purified pig brain GAD. Using similar assay, when we analyzed other type 1 diabetes patients with variable duration and age-onset, we found higher frequency of anti-GAD pos itivity in type 1 diabetes patients with older onset of age than in those with younger onset29). In the study of Kawasaki et al.15), this may lead to spurious increase in anti-GAD prevalence and titer in type 1 diabetes patients with ATD, who were older than those without ATD. From our study, we could not observe a higher prevalence of anti-GAD and ICA positivity in the type 1 diabetes patients with ATD than in those without ATD, who were not different in age at onset. We used the similar anti-GAD assay using the purified porcine brain GAD. Not the age at onset but the duration of type 1 diabetes was different. The difference in the prevalence of anti-GAD and ICA positivity between the two, however, reappears after adjustment for the duration. Type 1 diabetes patients with ATD had higher anti-GAD prevalences in longstanding type 1 diabetes compared with those without ATD. Furthermore, in short duration type 1 diabetes, they had lower prevalence of ICA compared with those without ATD. From these, we should be more careful for the possible confounders. This implies that the increase in polyendocrinopathy risks, represented by the increase in the prevalence of anti-GAD and/ or ICA, does not seem to be related only to the true association alone but also to the effect of the confounders.
Patients with ATD are known to predispose to develop other autoimmune disorders and present various organ-specific autoantibodies. Several studies reported that insulin autoantibodies (IAAs) were detected in 3–15% patients using competitive RIA14, 30). There are few reports concerning the presence of anti-GAD in non-diabetic patients with ATD31). There have been no reports regarding the difference in the prevalence or the titer of ICA512/IA-2 autoantibody. The titer of ICA512/IA-2 shown here in this study, decreased with duration of type 1 diabetes and thereby decreased in patients with ATD who had longer duration of disease. However, when we adjusted the duration of type 1 diabetes, we could not detect any differences either in the titer or the prevalence of ICA512/IA-2 between the two. Although the islet-specific autoantibodies frequently vary with the age and the duration of type 1 diabetes, this approach can bypass the spurious association. Likewise, what we can propose is that if we adequately control the duration of diabetes, type 1 diabetes patients with ATD are predisposed to have higher prevalences of anti-GAD antibodies, as previously suggested15, 16), but lower prevalence of ICA autoantibodies, when compared to type 1 diabetes patients without ATD.
Apart from ICAs, antibodies to GAD, IAAs, a novel islet antigen (37/40 kD antigen) are potential markers for type 1 diabetes16). ICA512/IA-2, which might be the antigen of 37/40 kD autoantibody was initially discovered by Rabin and coworkers8) with the screening of an islet cDNA library with sera from newly-onset type 1 diabetes patients. We have already indicated that not only ICA512/IA-2 but GAD was also one of the major antigens against ICA (unpublished observation) and there is strong concordance among ICA, anti-GAD and ICA512/IA-2 autoantibodies. However, we could not find a similar increase in the prevalence of ICA and ICA512/IA-2 in patients with ATD. Our type 1 diabetes patients were not onset cases, and this might contribute to the differential expression of anti-islet specific autoantibodies in type 1 diabetes patients with ATD. According to the study by M. Christie et al.16), anti-GAD prevalences were increased, the same as in this study, in type 1 diabetes patients with polyendocrine autoimmunity compared with those without. In contrast, antibodies to 37kD antigen, which is known to be the same antigen as ICA512/IA-2, were only detected in patients who developed acute-onset type 1 diabetes. Certain factors enhance antibody responses to GAD in type 1 diabetes with ATD, but antibodies to ICA512/IA-2 are more specific for type 1 diabetes and are useful serologic markers for acute-onset type 1 diabetes.
The broad concept of polyendocrinopathies takes into consideration that patients affected by at least one endocrine disease may also possess a positive serological reactivity against other endocrine cell targets12). Until now, a lot of studies aimed to investigate the nature of polyendocrinopathy. Several studies proved the clustering of ATD in type 1 diabetes patients and their family members32, 33). However, neither studies examined the degree of their coassociation precisely, nor have they investigated the possible cause. The present study was undertaken not only to determine the frequency of ATD amongst type 1 diabetes patients but also to study the possible cause for their clustering in patients with type 1 diabetes and their family members. It was attempted to investigate whether type 1 diabetes susceptible HLA DR/DQ alleles are shared between the type 1 diabetes patients with and without ATD to explain their coassociation. We could not find any differences in the distribution of HLA-DR and HLA-DQ alleles (or type 1 diabetes susceptibility alleles) and genotypes between the patients with and without ATD. Furthermore, when we measured HLA-DR/DQ polymorphisms in the first-degree family members of type 1 diabetes probands, three parental HLA DRB1/ DQA1/DQB1 haplotypes have been defined in the affected parent-offspring ATD families; DR4/DQA1* 0301/QB1* 0302, DR3/DQA1*0501/DQB1*0201 and DR9/ DQA1* 0301/DQB1*0303. All are the type 1 diabetes susceptible haplotypes in the Korean population (unpublished observation).
It has been hypothesized that the autoimmune endocrine diseases are each due to antigen-specific defects in suppressor T lymphocyte function. There is now increasing evidence in studies of autoimmune thyroid diseases and type 1 diabetes that suppressor cells are activated by irrelevant antigens but respond inadequately to specific relevant antigens11, 32). Further insults from the environment increase the deficiencies in regulatory cell activity, adding to the genetically induced dysfunction, specific defects in antigen presentation. The HLA system (essential for antigen precessing and presentation) is an important factor in the pathogenesis of these disorders. According to R. Volpe11), there is an organ-specific suppressor T lymphocyte defect in type 1 diabetes that is separate from that of ATD and the reduced activation of type 1 diabetes suppressor T lymphocytes by GAD is specific for this condition. The observation that type 1 diabetes is associated with ATD very closely and associated with similar HLA genes suggests a very similar pathogenesis for these entities and this might be a reason why they coassociate often. It may well be that each disease has separate genes and a separate organ-specific defect in immunoregulation. Probably, the defect is an organ-specific abnormality in suppressor T lymphocyte function, which is specific for each disease. The fact that some patients develop both of the diseases might be related to the inheritance of each closely related genes.
Acknowledgment
The authors extend thanks to the seoul type 1 diabetes registry for supplying Korean families. This stady was supported by a grant of the Korea health 21 R&D Project, ministry of Heslth 4 welfare, Republic of Korea(Hmp-00-B-21200-0039)