Multicentric Biatrial Myxoma in a Young Female Patient: Case Report
Article information
Abstract
We report a case of multicentric, biatrial cardiac myxoma in a 29-year-old female who complained of exertional dyspnea, abdominal distension and peripheral edema. Any other associated skin lesions, breast mass or endocrine disorder presenting complex form were not seen on her. Also, there was no contributory medical history, hypertension and diabetes mellitus. By using transthoracic echocardiography, we identified a biatrial myxoma attached to the interatrial septum. During surgical excision, we found a large right atrial myxoma with extension through the fossa ovalis into the left atrium and small myxoma attached to the right atrial free wall. After successful resection of interatrial septum and free wall, atrial septal defect was created during the resection and safely repaired by bovine pericardial patch.
INTRODUCTION
Primary myxoma is the most common type of cardiac tumor. In general, cardiac myxoma is sporadic and is commonly found in the left atrial cavity in middle-aged women. Biatrial myxoma is rare and, especially, multicentric myxoma is extremely rare. Myxoma needs an early diagnosis and urgent operation because of sudden death, embolization and systemic symptoms. We present a young female patient with multicentric biatrial myxoma who had no familial history. Surgical excision revealed a large right atrial myxoma with extension through the interatrial septum into the left atrium, and a tiny myxoma on the free wall of the right atrium. Atrial septal defects created during the resection were safely repaired using a bovine patch.
CASE
A previously healthy 29-year-old female presented with a four-week history of exertional dyspnea, nocturnal dyspnea, general weakness and abdominal distension. Physical examination revealed an acutely illlooking appearance. Blood pressure was 120/70 mmHg, pulse rate was 112 beats/min, temperature was 36.7°C, respiration was 24/min. A cardiologic examination revealed a middiastolic murmur with diastolic plop on the right lower sternal border and neck vein engorgement. Also, there were shifting dullness, ascites on abdomen and pitting edema on both lower extremities. The result of neurologic examination was normal. Laboratory examination revealed a normochromic, normocytic anemia with a hemoglobin of 9.8g/dL, a white blood cell count of 12,400 cells/dL with normal differential count and platelet count 318,000/uL. There was no specific abnormality of other laboratory tests. Chest radiography revealed cardiomegaly, and an electrocardiography showed sinus tachycardia and low voltage in limb leads. A computed tomographic scan of the brain and lung were normal. Two-dimensional transthoracic echocardiography revealed a 4×7 cm-sized right atrial myxoma arising from the atrial septum, which prolapsed through a normal-appearing tricupid valve with each cardiac cycle, and a 3×2cm sized left atrial myxoma. Also, there was a moderate amount of pericardial effusion(Figure 1). Early surgical intervention, utilizing cardiopulmonary bypass was done, and surgical approach to the tumor was biatrial in this patient. Surgical excision revealed two-lobulated 10×7×4 cm and 5×4×3 cm-sized large right atrial myxomas with extension through the interatrial septum into the left atrium, and there was 1×1×1 cm-sized tiny myxoma on the free wall of the right atrium which was not identified by transthoracic echocardiography. Also, there was a 5×4×2 cm-sized left atrial myxoma. Grossly myxoma was gelatinous, smooth and round with a glistening surface, friable and lobulated. The base of attachment of atrial myxoma was the fossa ovalis. The histologic findings of myxoma revealed the characteristic pale-staining, granular, inflammatory cells (Figure 2). Atrial septal defect created during the resection was safely repaired using a bovine patch. In postoperative follow-up, transthoracic echocardiography revealed no evidence of residual mass, shunt or valvular insufficiency. The patient was discharged unevently on the postoperative tenth day. With the familial tendency of occurrence in multicentric myxoma, members of her family were evaluated with transthoracic echocardiography but there was no intracardiac abnormal lesion.
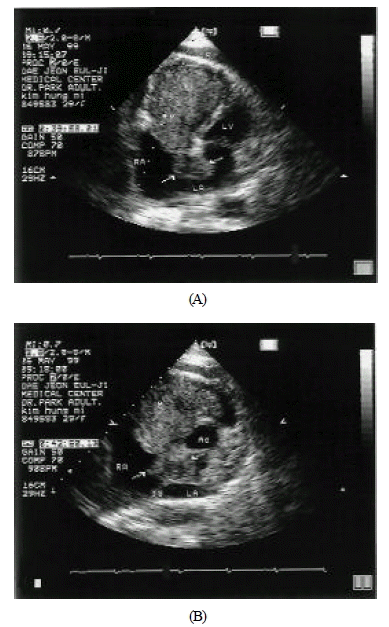
Transthoracic echocardiography revealed biatrial myxoma.
A. Apical four chamber view was demonstrating bilobulated 7×4cm-sized right atrial mass and 3×2cm-sized left atrial myxoma. The mass was prolapsed through the tricuspid valve.
B. Parasternal short axis view was demonstrating biatrial myxoma which was attached to septum.(M = myxoma, LV = left ventricle, RV = right ventricle, Ao = arota, RA = right atrium, LA = left atrium, and F = pericardial effusion)
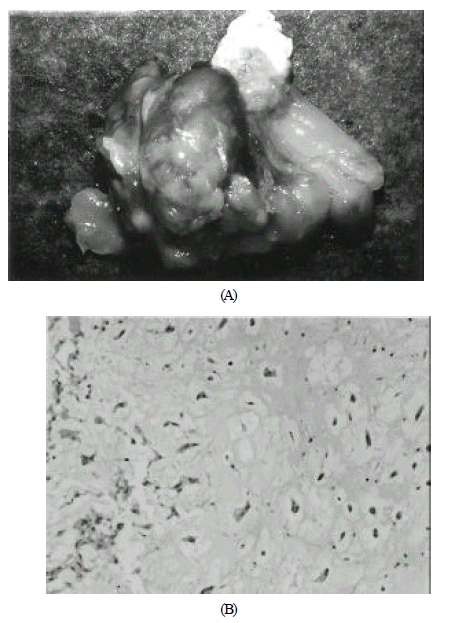
A. Gross appearance of biatrial myxoma with septum. Grossly myxoma was gelatinous, smooth and round with a glistening surface, friable and lobulated. The base of attachment of atrial myxoma was the fossa ovalis.
B. The histologic findings of myxoma revealed the characteristic pale-staining granular inflammatory cells. Myxoma cells are present with matrix, flanked by collagen zone(HE X400).
DISCUSSION
Primary heart tumors were found in 0.001 to 0.28 percent of autopsy cases1). Seventy-five percent of heart tumors are benign neoplasms. Cardiac myxoma, defined by two-dimensional echocardiography, represents approximately 50 percent of all benign cardiac tumors, with left atrial myxoma accounting for more than 70 percent2). The mean age of patients with sporadic myxomas is 56 years, and 70 percent are female.
Approximately 7 percent of cardiac myxomas are familial or part of the syndrome myxoma with complex abnormalities that also consist of myxomas in other locations (breast or skin), spotty skin pigmentation (lentigines, pigmneted nevi or both), and endocrine overactivity (pituitary adenoma, primary pigmented nodular adrenocortical disease or testicular tumor involving the endocrine component)6). In contrast to sporadic myxoma, familial or syndrome myxoma tumors tend to occur in younger individuals, are more often multiple in location and are more likely to have postoperative recurrences, probably reflecting their multicentric nature. Familial cardiac myxoma appear to have an autosomal dominant transmission, so routine echocardio-graphic screening of first-degree relatives is appropriate, particularly if the patient is young or has multiple tumors4–6).
Yipintsoi and associates described the first successful removal of a biatrial myxoma in 19677). Biatrial tumors are found in less than 5 percent of all myxoma cases8,9). Biatrial myxoma, as in this case, usually share a common site of attachment on opposite sides of the interatrial septum10,11). Most atrial myxoma arise from the area of the limbus of the fossa ovalis12).
The most common symptoms include generalized weakness, dyspnea on exertion, fever, arthralgia and palpitation. There is occasionally infected atrial myxoma17). Symptoms referable to myxoma include obstruction of flow, mechanical damage to valves, such as mitral stenosis, mitral insufficiency, supporting structures, embolization of tumor fragments or thrombus and constitutional complaints13,14). The embolization is dependent on motility and pedunculation of stalk. Movable and pedunculated tumor is likely to embolize15). Computed tomographic scan and/or MRI of the brain and lung is recommended for the evaluation of embolization if symptoms are clinically suspected.
Dato and colleague reported 24 percent of embolization in long-term results, and 78 percent of them were involved in the central nervous system16). Left atrial myxoma in 40–60 percent was more likely to embolization, which was through the patent foramen ovale. The presence of peripheral embolizaion without heart disease and atrial fibrillation in young patient is suspiect of cardiac myxoma7). But like this case, right atrial myxoma was much bigger than left atiral myxoma. We suggest that embolization is from the right atrium to the left atrium in this young female patient.
In 1945, Edler and Hertz firstly identified atrial myxoma by transthoracic echocardiograpy. Two-dimensional echocardiography provides substantial advantages over conventional M-mode echocardiography for the diagnosis and preoperative evaluation of intracardiac tumors. Especially, transesophageal echocardiography is the diagnostic modality of choice for atrial myxoma and is a more sensitive technique than transthoracic echocardiography15,17). (Diagnosis by transthoracic echocardiography:95.2%, transesophageal echocardiography: 100%, CT or MRI:70%, Identification of attachment point transthoracic echocardiography: 64.5%, transesophageal echocardiography:95.2%).
Differential diagnosis of benign and malignant cardiac tumors is possible by echocardiographic examination. Benign cardiac tumor shows smooth edge of base and homogeneous echogenicity, and malignant cardiac tumors reveal poorly defined, less rounded edges, no discrete capsule and more containing materials of cystic degeneration9).
Once the diagnosis is made, urgent excision of the myxoma is essential. Treatment is early surgical excision to prevent embolization, sudden cardiac death and to relieve symptoms. Surgical excision utilizing cardiopulmonary bypass is indicated and is generally curative. Cardiac myxoma recur in approximately 12 to 22 percent of familial cases and in about 1 to 2 percent of sporadic cases; tumor recurrence is most likely due to multifocal lesions in the former and inadequate resection in the latter3). With complete resection of atrial myxoma the recurrence rate is less than 5 percent.
The etiology of recurrence is incomplete, inadequate resection of tumor, spreading of tumor, multifocal occurrence and regrowing of tumor at the previous site1,3). However, the risk is higher in young patients and in familial, multiple, repeated and/or the so called complex myxoma. The multigrowth potential of the tumors seems more important than an inadequate surgical resection. As in this case, multicentric myxoma has a tendency to recur, so annual follow-up examination is needed. In general, is the case of cardiac myxoma, annual echocardiographic examination is recommenped for 3 years postoperatively, and thereafter if recurrence is clinically suspected18).