A Case of BOOP Developed during Bucillamine Treatment for Rheumatoid
Article information
Abstract
We describe a patient with rheumatoid arthritis(RA) who developed bronchiolitis obliterans organizing pneumonia(BOOP) during the treatment of bucillamine. A 51 year-old man was admitted to the hospital for an abnormal shadow on his chest radiogragh. He had been diagnosed as having RA 3 years previously and had been receiving 200 mg of bucillamine for 21 months. Two months prior to admission, he presented with a cough and his chest X-ray showed opacities in both lower lungs. He was treated with antibiotics for 2 months after the development of cough and lesions on the chest X-ray, but the symptoms and lung lesions became more aggravated. On admission, an HRCT revealed airspace consolidations in the subpleural space of both basal lungs and a CT-guided fine needle aspiration biopsy showed Masson’s body filling air space, interstitial infiltration of acute and chronic inflammatory cells and type II cell hyperplasia, consistent with BOOP. Bucillamine was stopped and 50 mg of prednisolone was administered. His symptoms and infiltrations on the chest X-ray resolved. We suggest that bucillamine should be considered as a drug possibly associated with BOOP.
Introduction
Bucillamine is a disease modifying antirheumatic drug (DMARD) which is used for the treatment of rheumatoid arthritis(RA) and shows clinical efficacy in RA1).
Bronchiolitis obliterans organizing pneumonia(BOOP) is a clinicopathologic syndrome of pulmonary responses which has become increasingly recognized and has been described in association with a variety of disorders and drugs2). BOOP has been reported in RA3,4) and in use of DMARDs5). Various side effects have been associated with bucillamine6,7), but to the best of our knowledge, until recently there has been no report on an association between BOOP and bucillamine. We describe a patient with RA who developed BOOP during the treatment of bucillamine.
CASE REPORT
A 51-year-old man was admitted to Korea University, Guro Hospital due to an abnormal shadow on his chest radiogragh. He had been diagnosed as having RA 3 years previously and had been receiving 200 mg of bucillamine for 21 months. Two months before admission, he presented with a cough and his chest X-ray showed opacities in both lower lungs. He was treated with antibiotics for 2 months after the development of a cough and lesions on a chest X-ray, but the symptoms and lung lesions became more aggravated.
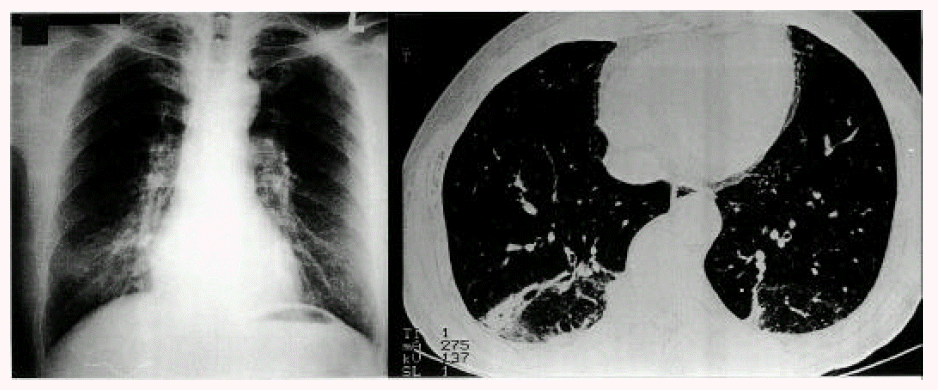
On admission, he was receiving 200 mg of bucillamine for the treatment of RA. He was a nonsmoker with no history of pulmonary disease or drug allergies. His blood pressure was 100/60 mmHg, pulse rate 64/min, respiratory rate 20/min, and temperature 36.8°C. Both proximal interphalangeal joints showed mild tenderness without swelling and his functional class of RA was grade I. Crackles were present in both lung bases. A chest radiograph revealed focal patch opacities in both lower lungs. Laboratory studies showed a white blood cell count 8,300/mm3, hemoglobin 12.5 g/dL, AST 26 IU/L, ALT 21 IU/L, ALP 69 IU/L, BUN 17.2 mg/dL and Cr 0.8 mg/dL. Antinuclear antibodies, ASLO, VDRL, HBsAg, HBsAb and anti-HCV were all negative. However, the erythrocyte sedimentation rate was 47 mm/hour, C-reactive protein was 14.2 mg/l and the rheumatoid factor was positive at 248.6 IU/ml. A pulmonary function test revealed forced vital capacity of 4.36L (94%), forced expiratory volume in 1 second of 3.12L (92%), FEV1/FVC of 99% and DLCO of 22.1 mL/min/mmHg (93%). A chest radiograph and HRCT revealed focal airspace consolidations in the subpleural space of both basal lungs (Figure 1). A computed tomography (CT)-guided needle aspiration biopsy showed Masson’s body filling air space, interstitial infiltration of acute and chronic inflammatory cells and type II cell hyperplasia, consistent with BOOP (Figure 2). No infectious agent was identified. He was diagnosed with BOOP possibly associated with bucillamine in RA. Bucillamine was discontinued and 50 mg of prednisolone was administered. His symptoms, chest X-ray and HRCT showed marked improvement (Figure 3). After discharge, he was on a tapering dose of prednisolone.

Chest radiograph and HRCT on admission showing focal patchy airspace consolidations in the subpleural space of both basal lungs.

Needle aspiration biopsy demonstrating Masson’s body filling air space, interstitial infiltration of acute and chronic inflammatory cells and type II cell hyperplasia, consistent with BOOP.
DISCUSSION
BOOP is a clinicopathologic entity of unknown origin characterized by the following:(1) clinical presentation associated with a preceding flu-like illness, (2) patchy infiltrates on a chest roentgenogram and/or computed tomographic (CT) scan, and (3) the pathohistologic pattern of intraluminal organization predominantly within the alveolar ducts8–10). BOOP has been observed in the context of connective tissue diseases and drugs such as gold, cephalosporin, sulfasalazine, D-penicillamine and infection5,8).
Bucillamine(N-(mercapto-2-methylpropionyl)-L-cysteine), a synthetic sulfhydryl (SH) compund like D-penicillamine, is a DMARD derived from thiopronin in 1987 in Japan, whose structure is analogous to D-penicillamine11). Bucillamine is one of the effective DMARDs, but bucillamine has been associated with various side effects such as proteinuria12), membranous glomerulonephritis13), agranulocytosis14), pemphigus-like skin lesions15) and lung injury16). Since the chemical structure of bucillamine is similar to that of D-penicillamine, side effects of bucillamine may be similar to those of D-penicillamine. However, to our knowledge there was no report on an association between bucillamine and BOOP, unlike D-penicillamine.
The lung is the most common site of extra-articular involvement in RA, which may cause a variety of pleuropulmonary disorders, including pleural disease, nodules, Caplan’s syndrome, pulmonary vasculitis, interstitial fibrosis and BOOP4). In this case, the patient was receiving bucillamine for the treatment of RA. BOOP developed during bucillamine treatment for RA and the lung lesion improved after discontinuation of the drug. Therefore, it is suggested that bucillamine is associated with the development of BOOP. This is the first report to suggest the possible association of bucillamine with BOOP.
In conclusion, we present a case of BOOP developed during bucillamine treatment for RA and suggest that bucillamine should be considered as a drug possibly associated with BOOP.