Imipenem-Cilastatin versus Sulbactam-Cefoperazone plus Amikacin in the Initial Treatment of Febrile Neutropenic Cancer Patients
Article information
Abstract
The treatment of infectious complications in cancer patients has evolved as a consequence of the developments in the chemotherapy of cancer patients. In this prospective, randomized study, we compared imipenem-cilastatin and sulbactam-cefoperazone with amikacin in the empiric therapy of febrile neutropenic (<1000/mm3) patients with liquids and solid tumours. Of 30 evaluable episodes, 15 were treated with imipenem-cilastatin and 15 were treated with sulbactam-cefoperazone plus amikacin. 73% of episodes were culture-positive; gram-positive pathogens accounted for 62% of the isolates. Bacteremia was the most frequent site of infection. The initial clinical response rate for both regimens was 60% (p>0.05). No major adverse effects occurred.
This study demonstrated that imipenem-cilastatin monotherapy and combination therapy of sulbactam-cefoperazone plus amikacin were equally effective empiric therapy for febrile granulocytopenic cancer patients.
INTRODUCTION
The increasing use of more intensive chemotherapeutic regimens to achieve maximal antitumour activity has produced severe and prolonged neutropenia in many patients1, 2). Life-threatening infection is a significant complication in patients undergoing intensive myelosuppressive therapy for treatment of malignancy. Neutropenia was known almost 3 decades ago as a major predisposing factor for the development of infection in patients with cancer. Absolute neutrophil counts less than 1000 cells/mm3 are associated with over 70% of septic episodes in neutropenic host. Fatality rates range from 30% to 70%, depending on the degree and the duration of neutropenia2, 3). Early empiric therapy with broad-spectrum bactericidal antibiotics is now standard practice in treating the cancer patients with febrile granulocytopenia1, 4).
Imipenem is an antibacterial agent of the carbepenem class of beta-lactams with a very broad spectrum of activity that includes most gram-negative and gram-positive pathogens, including aerobes and anaerobes, and with marked activity against species producing beta-lactamases1, 5, 6). Sulbactam is a beta-lactamase inhibitor which has been combined with ampicilin and cefoperazone7). Sulbactam itself has limited antibacterial activity against some aerobic gram-negative bacilli (AGNB) which include non-aeruginosa Pseudomonas spp. and Acinetobacter spp8, 9). In combination with cefoperazone, it extends the spectrum of the latter antibiotic to some anaerobes, including Bacteriodes fragilis, and many beta-lactamase-producing AGNB (10). Aminoglycosides have played an important role, especially in the treatment of gram-negative rod bacteremia in these granulocytopenic patients. Aminoglycosides are rapidly bactericidal and show concentration dependent killing, a feature that favors regimens that achieved high peak serum concentrations11, 12).
Several studies regarding imipenem-cilastatin and sulbactam-cefoperazone plus amikacin as initial therapy for febrile neutropenic cancer patients had been reported6, 13–16).
The purpose of our study was to compare the efficacy of empiric therapy with imipenem-cilastatin monotherapy and the combination of sulbactam-cefoperazone plus amikacin in the treatment of presumed bacterial infection in neutropenic cancer patients.
PATIENTS and METHODS
We performed this study at Ondokuz Mayis University Hospital, Samsun. Patients were eligible if they had liquids or solid tumours, neutropenia (<1000/mm3) and fever (defined by at least two oral temperature readings above 38.0 °C at least 4 h apart within a 24-h period, single oral temperature above 38.5 °C) in the absence of an obvious noninfectious cause of fever, such as administration of blood products or cytotoxic drugs. The patients were informed about antibiotic regimens.
Initial assessment included history and physical examination, urinalysis, complete blood counts, absolute neutrophil counts, blood biochemistry and chest X-ray. Specimens for bacterial and fungal cultures were collected from nose, throat, urine, stool, sputum (if available), blood and any other appropriate sites before commencement of antibiotic treatment. The antimicrobial prophylaxis was not used before initial treatment. Fifteen patients were randomly allocated to receive either imipenem-cilastatin (500 mg i.v. every 6 h) or a combination of sulbactam-cefoperazone (2 g i.v. every 12 h) plus amikacin (15 mg/kg/day, on average 500 mg i.v., every 12 h).
The response to empiric therapy was evaluated at 72 h and therapy was modified only if the patient did not respond, or deteriorated, if there were adverse reactions, or if a resistant pathogen was isolated. If fevers persisted after five days of antibotic therapy, empirical antifungal therapy (fluconazole, 200 mg PO or IV every 24 hours) was started. Therapy was generally continued until the patient was free of symptoms of infection for 5 days or granulaocyte count increased to >1000/mm3.
Antibiotic-related nephrotoxicity was diagnosed when the serum creatinine level increased to 0.5 mg/dl from baseline in the absence of other causes of renal dysfunction or other nephrotoxic drugs. Antibiotic-related hepatotoxicity was indicated when the serum aspartate and alanine aminotransferase increased 2-fold from baseline in the absence of other causes of hepatic dysfunction or hepatotoxic drugs.
RESULTS
During a 12-month period, 30 patients with episodes of fever and granulocytopenia were enrolled in this study. Clinical characteristics of the evaluable patients are shown in Table 1.
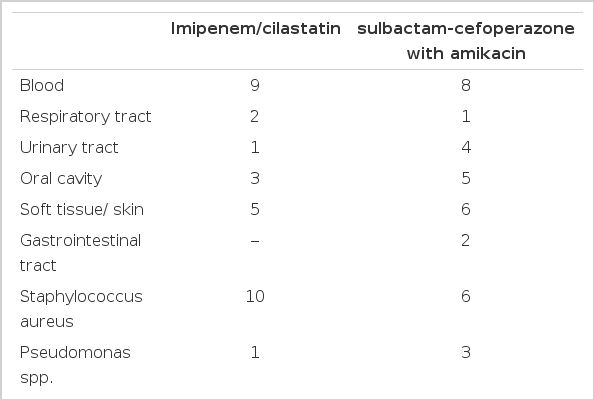
Of the 30 evaluable episodes, 15 were treated with an imipenem-cilastatin monotherapy and a combination of sulbactam-cefoperazone plus amikacin. Age and underlying disease were comparable. Numbers of culture-positive episodes were 10 and 11, respectively, in the two regimens and the overall rate of positive cultures was 73%. The sites of infection and the causative organisms are listed in Table 2.
An early clinical evaluation after 72 h of empiric therapy showed that 60% of patients responded favorably to the initial empiric therapy. Table 3 shows the status at early evaluation. All of the patients in whom culture-negative episodes were observed were successfully treated with the initial regimen. One patient who had herpetic infection used acyclovir.
There were no statistically significant differences between the treatment groups in patient outcome for the microbiologically documented, clinically documented, or possible infection groups (p>0.05).
In 9 (60%) of imipenem-cilastatin groups and 9 (60%) of sulbactam-cefoperazone plus amikacin groups, an outcome of success without modification of therapy was achieved. The addition of vancomycin was the most frequently used modification in both groups. There were no major adverse effects requiring a change of antibiotics. However, two patients developed drug induced diarrhea and emesis in the group receiving the imipenem-cilastatin. None of these was considered serious or severe.
DISCUSSION
Granulocytopenia has been closely associated with cancer and its treatment4, 17). Despite the poor inflammatory reaction in these patients, the onset of fever usually represents an infection in the body1, 13, 18). Confirmation of infection usually involves a delay of some days before all the laboratory results are available. The primary objective of empiric antibiotic therapy is to protect many patients with cancer from the immediate cause of death2, 18). In order to reduce infection-related morbidity and mortality, a number of treatment concepts have been utilized during the past 3 decades. Therefore, the antibiotic regimens must be selected against the major known pathogens1, 3, 18, 19).
Since the 1970’s, the most frequently used empiric antibacterial therapy has consisted of two or three-drug regimens, usually combining a cephalosporin, an antipsedomonal penicilin or both with an aminoglycoside1, 18). The rationale for this approach is based on the wide range of potential gram-positive and gram-negative organisms necessitating coverage and the evidence that combination therapy results in additive or synergistic bactericidal activity. The availability of newer, broader spectrum cephalosporins, carbapenems and monobactams has led to considerable controversy regarding the use of these agents as monotherapy for empiric antibacterial treatment of the febrile compromised host20, 21).
Most investigators have shown that antibiotic combinations of aminoglycoside with a beta-lactam for synergy and avoidance of the emergence of resistant bacteria are more effective than monotherapy in the treatment of febrile neutropenic patients22, 23). Monotherapy with imipenem-cilastatin has been previously demonstrated to be effective in noncomparative trials. Comparative studies with imipenem-cilastatin versus piperacillin plus amikacin and ceftazidime plus amikacin have shown that imipenem-cilastatin is equal to other combinations24, 25). These results have demonstrated that imipenem/cilastatin monotherapy could be a practical alternative regimen6, 13, 14, 24).
The incidence of infection due to gram-positive organisms has increased markedly in patients with cancer26, 27). In our study, 73% of the episodes had a positive culture; gram-positive pathogens accounted for 62% of the isolates. The response rate (60%) was the same for the two regimens. Each regimen covers the organisms most frequently isolated in neutropenic patients. Toxicity related to antibiotics were minimal in both groups.
In summary, this study demonstrated that monotherapy of imipenem-cilastatin and the combinations of sulbactam-cefoperazone with amikacin were equally effective in the treatment of febrile episodes in neutropenic cancer patients.