Electron Microscopic Evaluation of Adhesion of Helicobacter pylori to the Gastric Epithelial Cells in Chronic Gastritis
Article information
Abstract
Background
The adhesion of H. pylori to the gastric epithelial cells may be an essential step for the pathophysiology of various H. pylori-induced gastrointestinal diseases. The purpose of this study was to investigate the ultrastructural relation of H. pylori and gastric epithelial cells in their adhesion.
Methods
Endoscopic biopsy of gastric antrum and body was performed from 15 patients (9 men, 6 women) with chronic gastritis and H. pylori infection. The specimens were processed for electron microscopy and observed with a transmission electron microscope (Hitachi H-600).
Results
On the basis of morphological appearances, the adhesions of H. pylori to the gastric epithelial cells were categorized into three types; filamentous connection, adhesion pedestals and membrane fusion. Coccoid and undetermined forms adhered mainly by the filamentous connection, whereas the bacillary forms adhered primarily by the adhesion pedestals and membrane fusion.
Conclusion
Various types of adhesion were associated with H. pylori and gastric epithelium. Further studies are needed to evaluate the influence of different types of adhesion to the pathophysiology of H. pylori.
INTRODUCTION
Helicobacter pylori is a gram negative bacteria living in the stomach. It is typically curved bacilli in form, however the coccoid form is not rare1,2). The bacillary form is known to be active in the pathogenesis of various gastric diseases, although the roles of coccoid form are not clear yet. As known so far, the bacillary form can convert to the coccoid form in bad environmental conditions such as lack of nutrients3), increase of the intragastric pH4), exposure to antibiotics5), long-term culture6), etc.
Ultrastructural studies have been used to elucidate the structural characteristics of H. pylori3,7) and to clarify the pathophysiologic association between H. pylori and infected gastric epithelial cells8,9). Several investigators reported the adhesion of H. pylori to the gastric epithelial cells, suggesting such an adhesion may be closely related to the pathogenesis of H. pylori-related gastric diseases10,11). Also, the frequent presence of coccoid forms near the severely damaged gastric epithelial cells suggests the morphological changes of H. pylori could be associated with the damages of the gastric epithelium10,11).
This study aimed to evaluate the ultrastructural features of adhesion between the H. pylori and infected gastric epithelial cells as a way to understand the pathophysiology of H. pylori.
MATERIAL AND METHODS
1. Study Population
Fifteen patients (9 males and 6 females; average age 51) with chronic gastritis were selected for this study among those who visited the Department of Internal Medicine with upper gastrointestinal symptoms. They had no prior history of taking bismuth, H2 antagonists, proton pump inhibitors or antibiotics for at least 4 weeks at their first visits to the hospital. The Urea Breath Test was performed and the presence of H. pylori was confirmed in the biopsy.
2. Electron Microscopy
Biopsies from the gastric antrum and corpus were performed during the diagnostic endoscopy of the upper gastrointestinal tract. The collected tissues were fixed in 2.5% glutaraldehyde, 0.1 M phosphate buffer (pH 7.4) and postfixed in 1% osmium tetroxide. After dehydrating with an ascending series of ethanol, the tissues were embedded in the EMbed-812. Sections were obtained with an ultramicrotome, double-stained with uranyl acetate and lead citrate and observed with H-600 transmission electron microscope (Nissei Sangyo, Japan) at the acceleration voltage of 75kV.
3. Analysis
All H. pylori attached to the surface of the gastric epithelial cells at a single thin section from each patient were photographed at ×10,000–12,000. Firstly we analyzed the morphology of H. pylori and the types of adhesion between H. pylori and the gastric epithelial cells. Then, the adhesion was analyzed by counting the number of H. pylori according to the types of adhesion.
RESULTS
1. Morphology of H. pylori
Based on the appearance on the sections, H. pylori was categorized into three types; 1) a bacillary form with rod or curved shapes 2) a coccoid form with a round shape and 3) an undetermined form with an intermediate morphology between bacillary and coccoid forms. No ultrastructural difference was observed between different types of H. pylori (Figure 1).
2. Adhesion Types
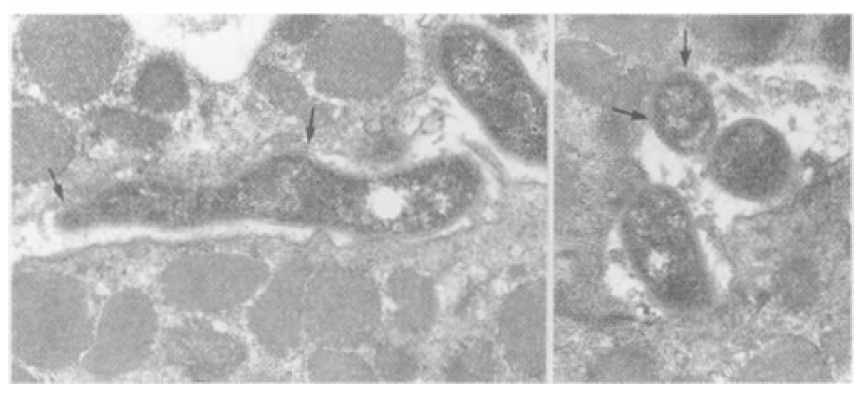
In this study, we defined the adhesion as an attachment of H. pylori to the gastric epithelial cells. Depending on the structures intervened, three types of adhesion were observed. In a filamentous connection, H. pylori was attached to the gastric epithelial cell via thin filaments (Figure 2). Adhesion pedestals; the elevated membrane thickening of gastric epithelial cells at the site of contact characterized some adhesion (Figure 3). Sometimes, H. pylori and the gastric epithelium were completely fused not to allow discretion, which was defined as a membrane fusion (Figure 4). Also, depending on the relative location of the H. pylori-attaching part with a reference to the virtual straight line connecting the superior border of the gastric epithelial celi, each type was divided into 2 patterns; 1) with membrane depression located below the line and 2) without membrane depression located above the line.

Examples of H. pylori attached to the gastric epithelium via filamentous connection (arrow). A bacillary form of H. pylori (A) is located on the surface and a coccoid form (B) is in the depression of the gastric epithelial surface.

A bacillary form (A) and a coccoid form (B) of H. pylori are attached to the gastric epithelial surface via adhesion pedestals. The attaching part of the epithelium is thickened and elevated (area between 2 arrows).
3. Relation of H. pylori and Adhesion Types
We summarized the observed number of different forms of H. pylori attached to the gastric epithelial cells depending on types of adhesion in Table 1. Coccoid and undetermined forms were attached mostly by filamentous connections, whereas the bacillary form was attached mostly via adhesion pedestals and filamentous connections (Table 1).
DISCUSSION
Since the first identification and isolation of H. pylori from the human body by Drs. Marshall and Warren12), H. pylori has been known to be a main factor for various upper gastrointestinal diseases, such as chronic gastritis, peptic ulcer, B cell lymphoma and gastric cancer. Many studies to elucidate the pathophysiologic roles of H. pylori are being progressed13). Ultrastructural analysis with an electron microscopy can be a useful method of studies to observe the direct relationship of H. pylori to the gastric epithelium and to understand the pathologic changes of the gastric epithelium3,8,14,15).
In an ultrastructural analysis, Smoot et al.16) reported that in culture the degenerative changes of gastric epithelial cell membrane and the extinction of microvilli appeared with time. They also reported that H. pylori attached to the elevated part of gastric epithelial cells at the site of disappeared microvilli, which involves the actin polymerization16). Similar involvement of actin polymerization in the adhesion was reported in E. coli’s adhesion to intestinal epithelial cells17). Different types of adhesion have been reported to be associated with the decrease in microvilli and the changes of cytoskeletons1,18,19). It has been reported that glycocalyx is formed on the surfaces of gastric epithelial cells and H. pylori and mediates adhesion, especially in the form of filaments during the initial stage of adhesion, which is shown by lectin binding2,20).
The standard classification for adhesion types has not been suggested yet. Noach et al.8) classified the adhesion into 5 types; adhesive pedestals, membrane depression, abutting adhesion, membrane fusion and internalization, and reported the abutting adhesion as the most common type among them. During the analysis based on Noach et al’s classification8), we observed that the membrane depression and abutting adhesion were actually present, mixed with adhesion pedestals and membrane fusion. This led us to classify the adhesion from a new point of view. As written in the result section, we classified the adhesion broadly into 3 types, depending on the structures involved and subdivided each type into 2 patterns according to the membrane depression. The internalization in Noach et al’s classification8) was included in the pattern of “with membrane depression”, because H. pylori observed in the gastric epithelial cells in this study were not present in the cytoplasm but in a vacuole-like structure thought to be a part of the deep invagination from the cell membrane. Putting aside the question whether H. pylori really invade the cell or not, the authors think that this pseudointernalization may be related to the same mechanisms that cause the membrane depression.
Even though direct relationship of different types of adhesion to the pathophysiology of H. pylori has not been proved, as far as we know, several evidences suggest the possibility that the different type of adhesion is not just a different morphological relation between H. pylori and the gastric epithelium, but a phenomenon showing a progress to cause some diseases1,2,11,16,18–20).
Hessey et al.11) reported that the involution of cells was accelerated when the contact areas between H. pylori and gastric epithelium was wider. From this observation, they emphasized that the adhesion between H. pylori and the gastric epithelium should be understood as a pathophysiologic feature of H. pylori, not as a simple physical contact and suggested the possibility that H. pylori may accelerate the damage of the gastric epithelium through such types of adhesion as abutting and membrane depression11). Our results showed that H. pylori attaches the gastric epithelium most frequently by filamentous connections, followed by adhesion pedestals and membrane fusions. If Hessey et al’s claim11) is correct, the adhesion pedestals without membrane depression in our report could be a beginning stage of the pathologic process leading to chronic gastritis. It is thought that further studies regarding the types of adhesion and the degree of cellular damage are necessary to make clear this point.
There are still arguments whether different morphology of H. pylori has any effect on the adhesion. Janas et al15) reported the they observed no coccoid form at adhesion pedestals nor on any other adhesion sites. In this study, we observed lots of coccoid form of H. pylori adhered to the gastric epithelial cells, which was contrary to Janas et al’s observation15). It may be argued that many of the coccoid forms observed in this study can be artifacts due to sectional direction and we did not check this possibility by sectioning the specimen serially or examining by scanning electron microscopy. However, considering that almost the same numbers of attached coccoid and bacillary forms were observed in this study, we believe that a considerable number of coccoid forms attached to the gastric epithelial cells.
Our results showed that the coccoid forms adhere mainly by the filamentous connection, while the bacillary forms use adhesion pedestals and filamentous connection for adhesion. The adhesion of undetermined forms to the gastric epithelial cells was similar to the coccoid forms, in that they mainly attach to the gastric epithelial cells through the filamentous connection, but not through adhesion pedestals. Also, these undetermined forms have ultrastructural features not different from either bacillary or coccoid forms. Several reports suggested the ultrastructural changes of H. pylori during conversion from the bacillary to the coccoid forms15,21,22). These suggest that the undetermined forms in our study contain a considerable number of intermediate forms, not only in shape but also in behavior, between bacillary and coccoid forms, as well as some obliquely sectioned bacillary forms. Even with the structural differences from earlier reports15,21,22), the possibility that the undetermined form may represent a group of H. pylori changing their shapes from the bacillary into the coccoid still needs to be explored.
In conclusion, the results of this study suggest, based on the number of adhesion patterns observed, that the adhesion of H. pylori to the gastric epithelium might proceed in the order of the filamentous connection, adhesion pedestal formation and finally, membrane fusion. However, the possibility that different types of H. pylori may affect the gastric epithelium in different ways to form different adhesion structures, such as the filamentous connection by the coccoid form or the adhesion pedestal formation by the bacillary form, should be verified in further studies.
Acknowledgements
This study was supported in part by the 1999 Research Grant of Medical Science Research Center of Korea University to HJC. HJC and C-SU are supported by the Brain Korea 21 Project of the Ministry of Education and Human Resources Development, Republic of Korea. We thank Ms. Eun Kyung Park for technical assistance.