Role of TGF-β 1 and TGF-β Type II Receptor in Gastric Cancer
Article information
Abstract
Background:
TGF-β is known as a cell growth inhibitory factor to suppress almost all cells, including the epithelial cell. Unlike normal cells, cancer cells are not affected by TGF-β growth inhibitory action and the lack of TGF-β receptor expression or mutation is being reported as its mechanism, which is rarely studied in Korea. Therefore, we investigated this study to clarify the role of TGF-β I and TGF-β II receptors in gastric cancer.
Methods :
23 cases that underwent operations for gastric cancer provided RNA collected from their carcinoma tissues and adjacent normal tissues. We investigated the level of TGF-β 1 and T β R-II mRNA expression with semi-quantitatively reverse transcription PCR and analyzed the correlation with prognostic factors, such as tumor size, depth of invasion, tumor differentiation and lymph-node metastasis.
Results :
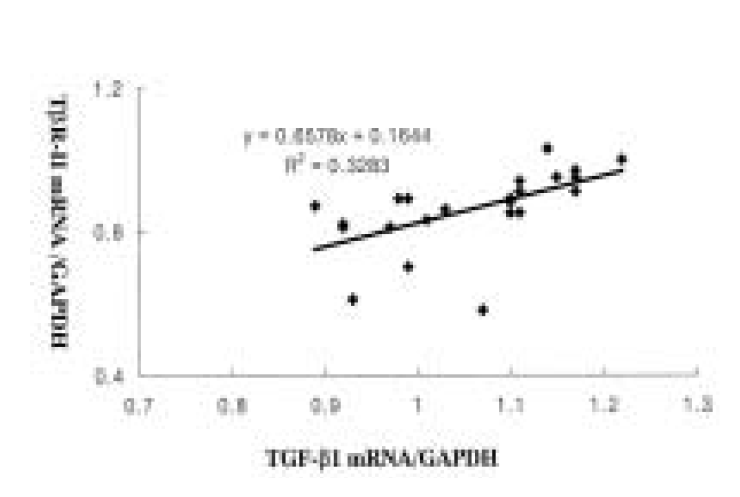
(1) TGF-β I and T β R-II mRNA were expressed in all carcinoma tissues and adjacent normal tissues of the 23 cases without statistical difference in the level of the expression. (2) The level of TGF-β 1 mRNA expression was higher in patients with gastric cancer invaded only at the mucosa and submucosa than in patients with gastric cancer invaded over muscular propria, and also higher in the patients without lymph-node metastasis or perineural invasion than in the patients with lymph-node metastasis or perineural invasion. There was no significant correlation between the level of T β R-II mRNA expression and several parameters, such as age, gender, tumor size, location, differentiation, Lauren’s classification and vascular invasion. (3) There was a significant correlation between the level of TGF-β 1 and T β R-II mRNA expression in carcinoma tissues.
Conclusion :
It indicated that TGF-β 1 mRNA expression in gastric cancer might concern the early stage of gastric carcinogenesis and, unlike the earlier reports, it was higher in patients with early gastric cancer, negative lymph-nodes or negative perineural invasion. Further studies are required to clarify the role of TGF-β 1 in gastric carcinogenesis with more patients.
INTRODUCTION
Transforming growth factor-beta (TGF-β) is dimeric polypeptide of 25 kDA of molecular weight, known as a potent growth inhibitor of most cells, including epithelial cells1,2). 3 isoforms (TGF-β 1, TGF-β 2, TGF-β 3) are in a human body and TGF-β 1 plays the most important role, because of which it is being studied most3,4). TGF-β 1 is combined with TGF-β 1 receptor and TGF-β type II receptor (T β R-II) to form heteromeric receptor complex for effect5). Carcinoma cells, unlike normal cells, are not affected by growth inhibiting action of TGF-β 1. It is recently proved by low expression of TGF-β receptor or mutation6,7).
Patients with advanced gastric cancer were tested to identify the presence of TGF-β 1 mRNA through endoscopic biopsy. In comparison with the survival rate after operation, TGF-β 1 mRNA positive patients group showed a low rate compared to TGF- β 1 mRNA negative group, because of which it is reported as an important preoperative prognostic indicator in advanced gastric carcinoma8). TGF-β Type II receptor expression is associated with a poor prognosis in the progression of gastric cancer but it is rarely studied9).
To this end, we investigated the correlation between prognostic indicators of gastric cancer, such as invasiveness of gastric cancer, lymph-node metastasis, perineural and vascular invasion, and TGF-β 1 and T β R-II mRNA.
PATIENTS and METHODS
1. Patients
23 cases who underwent operation for gastric cancer at our hospital from June, 1999 to Oct., 1999 were studied. Of 23 patients, 18 were in the early stage of gastric cancer, 5 in the progression of gastric cancer. They were 14 males and 9 females with the mean age of 59.
2. Methods
1) Specimen collection and mRNA extraction
Tissues were collected around the carcinoma part and the normal part furthest from the carcinoma right after gastric resection, and frozen in liquid nitrogen for reverse transcription PCR (polymerase chain reaction) before storage at −80°C. Tissues stored in liquid nitrogen were crushed into fragments and mRNA was extracted by using mRNA extraction kit (Quiagen GmbH, Hilden, Germany).
2) Reverse transcription PCR and Quantitative analysis of TGF-β 1, T β R-II mRNA
Random hexamer was used as a primer to react dNTP mixture with MMLV only to get cDNA. And then, TGF-1 (5′CCAGCCTGAGGCCGACTACT-3′), TGF-β type II receptor (5′-CAACAACATCAACCACAAC-3′) and GAPDH (5′-ACCACAGTCCATGCCATCAC-3′) went through PCR with each specific primer. TGF-β 1 PCR followed a cycle of denaturation at 95°C for 60 sec., annealing at 56°C for 60 sec. and extension at 74°C for 120 sec. The total cycle number was 35 times. TGF-β type II receptor PCR followed a cycle of denaturation at 95°C for 60 sec. annealing at 58°C for 60 sec., and extension at 74°C for 120 sec. The number of total cycles was 35 times. GAPDH PCR had a cycle of denaturation at 94°C for 60 sec., annealing at 60°C for 90 sec. and extension at 74°C for 120 sec. The number of total cycles was 35 times. PCR product was treated by the electrophoresis method in 1% agarose gel, including ethidium bromide, to confirm the expression of TGF-β 1, T β R-II mRNA and GAPDH. TGF-β 1 and T β R-II mRNA expression was measured semi-quantitatively by comparative RT-PCR, and GAPDH was used as house-keeping gene. GEL-DocTM single wave length minitransilluminator was used for analysis.
3) Correlation with prognostic indicators of gastric cancer
We analyzed the correlation between the possibility of prognostic indicators of gastric cancer, such as tumor size, depth of invasion, location, tumor differentiation, Lauren’s classification, lymph-node metastasis, perineural and vascular invasion, and TGF-β 1 and T β R-II mRNA expression.
3. Statistical Analysis
Mann-Whitney U test was used to compare TGF-β 1 and T β R-II mRNA expression. Spearman test was used to check the correlation of TGF-β 1 and T β R-II mRNA expression in carcinoma tissues. p<0.05 was regarded as statistically significant.
RESULTS
1. TGF-β 1 and T β R-II mRNA expression in carcinoma tissues and in adjacent normal tissues of gastric cancer
All 23 patients showed TGF-β 1 and T β R-II mRNA expression in carcinoma tissues and in adjacent normal tissues of gastric cancer. Average TGF-β 1 mRNA/GAPDH ratio (mean±SD) was 1.06±0.02 in carcinoma tissues and 1.07±0.03 in adjacent normal tissues, which showed no difference between two groups. Average T β R-II mRNA/GAPDH ratio (mean±SD) was 0.86±0.02 in carcinoma tissues and 0.88±0.02 in the adjacent normal tissues, which displayed no difference between two groups (Figure 1).
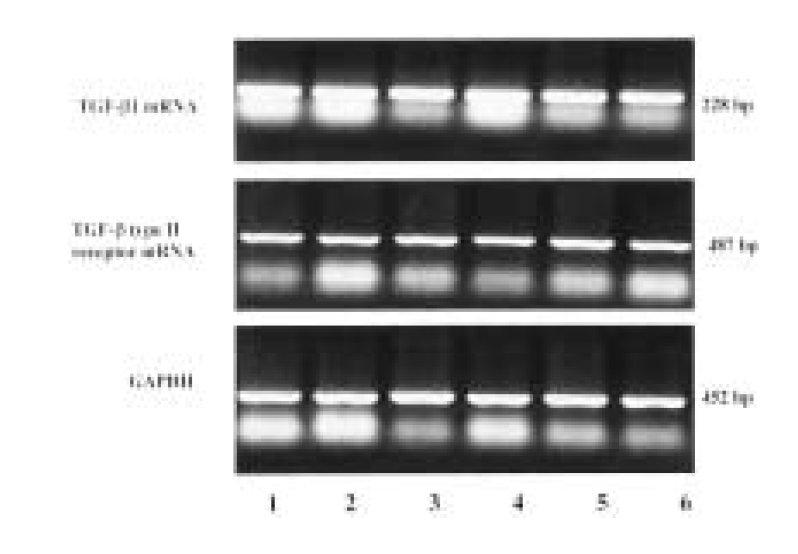
TGF-β 1 and TGF-β type II receptor (T β R-II) mRNA expression in carcinoma tissues and in adjacent normal tissues of gastric cancer by comparative RT-PCR. Glyceraldehyde 3-phosphate-dehydrogenase (GAPDH) hybridization to the same blots is shown as a loading control. Lane 1, 3, 5, carcinoma tissue; Lane 2, 4, 6, adjacent normal tissue.
There was a relatively significant correlation between the level of TGF-β 1 and T β R-II mRNA in carcinoma tissues (R=0.57, p<0.05, Figure 2).
2. Relationship between prognostic indicators of gastric cancer and the level of TGF-β 1 mRNA expression
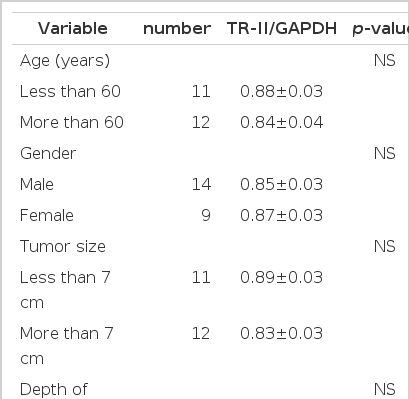
The difference in the level of TGF-β 1 mRNA according to age and gender was not displayed (Table 1). TGF-β 1/GAPDH ratio in the group of carcinoma only at the gastric mucosa or submucosa and in the group of carcinoma invaded over muscular propria was 1.08±0.02 and 0.98±0.03, respectively, and TGF-β 1 mRNA expression increased in the mild invaded group. When compared according to lymph-node metastasis or perineural invasion, the group without lymph-node metastasis (1.13±0.01) and the group without perineural invasion (1.08±0.02) showed TGF-β 1 mRNA expression more increased than the group with lymph-node metastasis (0.97±0.02) and the group with perineural invasion (0.97±0.02) (p<0.05). But TGF-β 1 mRNA expression was not different according to tumor size, location, tumor differentiation, Lauren’s classification and vascular invasion (Table 2).
3. Relationship between the level of prognostic indicators of gastric cancer and T β R-II mRNA expression
T β R-II mRNA/GAPDH ratio in the cancer group only at the mucosa or submucosa and in the cancer group invaded over the muscular propria was 0.88±0.03 and 0.79±0.05, separately. The tumor size groups below 7 cm and more than 7 cm showed 0.89±0.03 and 0.83±0.03. The groups without and with lymph-node metastasis showed 0.93±0.03 and 0.84±0.03. It displayed that T β R-II mRNA expression increased in the group of mild invasion and small tumor size and without lymph-node metastasis, but it was not statistically significant (Figure 2). The difference in the level of T β R-II mRNA expression was not observed according to age, gender, location, tumor differentiation, Lauren’s classification, vascular and perineural invasion.
DISCUSSION
TGF-β is one of cell regulators that influence various cell functions and plays an important role in cell differentiation, inflammatory response, immunologic function and carcinogenesis10,11). Such various functions of TGF-β work when they combine with the cell surface receptor of various structures for signal communication from outside to inside of cells12,13) TGF-β is known to inhibit the growth of almost all the cells, including the epithelial cell, but, unlike normal cells, cancer cells are not affected by the growth inhibitory function of TGF-β, which is explained as the decreased expression of TGF-β receptors or mutation6,7)
According to the earlier reports about solid tumor like pulmonary cancer14), breast cancer15,16), colorectal cancer17), pancreatic cancer18), TGF-β expression is known as a poor prognostic factor because it is well associated with invasiveness of cancer cell, invasive metastasis, disease progression and decreased survival. It is proved that the cancer cell resists against the growth inhibitory function of TGF-β only to stimulate neovascular composition and change the immunologic function to cell matrix or human cancer cell, which increases invasiveness and remote metastasis of cancer cells. TGF-β expression increased in gastric cancer like other solid tumor19–22). Especially, it was observed in the group with lymph-node metastasis, poor differentiation, disease progression and short survival18,23–25)
The biological activation of TGF-β begins when TGF-β ligand combines first with TGF-β type II receptor and then with TGF-β I receptor to form the heteromeric receptor complex consisting of TGF-β ligand-TGF-β I and II receptors. TGF-β Type III receptor indirectly works only at the signal transduction without self-signal communication13,26). It is reported that the loss of TGF-β receptors expression in prostatic cancer correlates with poor prognosis, whereas TGF-β expression in pulmonary cancer is associated with poor prognosis. The role of TGF-β receptors in malignant tumor has not been clarified yet27,28). According to the report about TGF-β receptor expression in gastric cancer, TGF-β type II receptor expression is not related with invasiveness of gastric cancer, lymph-node metastasis and survival rate, but with poor prognosis in the progression of gastric cancer8).
But this study observed a different result from the existing reports that TGF-β 1 mRNA expression increased in the group with mild invasiveness of gastric cancer and without lymph-node metastasis or perineural invasion. Though there was no statistically significant difference, T β R-II mRNA expression increased in the group with mild invasiveness, small tumor size and without lymph-node metastasis.
Such a contrary result can be explained with the following possibilities. First, Fowlis et al. reported that TGF-β RNA and TGF-β protein expression types are not coincident in skin by chemical carcinogen29). Cut et al. reported that lack of TGF-β 1 expression at the stage of post-transcription may provoke the growth of cancer cell in case of advanced malignant tumors30). This study observed only at the level of mRNA I reverse transcription PCR, which means there is a good possibility of a change in the post-transcriptional stage, including protein level. Second, in vitro experiment with a colon carcinoma cell line and gastric carcinoma cells displayed TGF-β growth inhibition on such carcinoma cells, which is contrary to a report that it is related with invasiveness of cancer cell and remote metastasis31,32). It explains that in vivo cancer cell growth is decided not only by TGF-β but also by interaction with various cell regulators (cytokines). Also, TGF-β may cause a contrary result depending upon circumstances. Third, as the relation of TGF-β expression and prognosis turned out differently in prostate cancer and pulmonary cancer, the roles of TGF-β and TGF-β receptor in malignant tumors have not been clarified yet27,28). Fourth, since Sporn et al. suggested that a defect in TGF-β receptor system can cause lack of TGF-β growth inhibitory action, it has been reported that mutation of TGF-β type II receptor is associated with lack of TGF-β growth inhibitory action in some carcinomas and transfection of wild-type TGF-β Type II receptor to them recovers sensitivity to TGF-β to reduce the cancer cell line. It means the resistance to TGF-β is connected not with the lower volume of TGF-β type II receptor but with the structural defect by mutation. This study considered only a quantitative relation of TGF-β I and TGF-β type II receptors, which might produce a different result7,33–36).
Finally, TGF-β 1 and T β R-II mRNA expression in carcinoma tissues and adjacent normal tissues are not different, which means that T β R-II mRNA expression may be engaged in the early stage of gastric carcinogenesis. Unlike the earlier reports, it was observed that TGF-β I mRNA expression increased in the group with mild invasiveness of gastric cancer and without lymph-node metastasis and perineural invasion, but it was a limited study with only a few patients. Therefore, it is considered that the study should be continued with more patients to conclude the role of TGF-β I in gastric carcinogenesis.