INTRODUCTION
Eosinophilic granulomatosis with polyangiitis (EGPA) is one of the three subtypes of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV). The histological features of EGPA are characterised by necrotising vasculitis in small-sized vessels, including capillaries and their adjacent arterioles and venules, with evidence of eosinophil infiltration and granuloma formation [
1,
2]. However, even though EGPA is included as a subtype of AAV, ANCA is only found in approximately 40% of patients with EGPA [
3]. Thus, ANCA positivity is not essential in classifying EGPA according to the 1990 American College of Rheumatology (ACR) criteria (the 1990 ACR criteria) [
4] and the 2007 European Medicine Agency (EMA) algorithm [
5]. The clinical features of EGPA patients with and without ANCA are different: EGPA patients with ANCA predominantly exhibit vasculitic features and those without ANCA preferentially have allergic features [
6,
7].
In patients with AAV, detection of ANCAs directed against myeloperoxidase (MPO) and proteinase 3 (PR3) (MPO-and PR3-ANCA) in the blood is a typical laboratory feature. Notably, there is accumulating evidence that suggest that different phenotypes and prognoses are expected based on ANCA types [
8]. Similarly, we have previously shown that AAV patients harbouring MPO-ANCA, PR3-ANCA, and ANCA-negativity revealed significantly distinct clinical features [
9]. However, the clinical impact of ANCA types in AAV subtypes, especially EGPA, is not well understood. Recently, a study investigating the clinical characteristics of PR3-ANCA positivity in EGPA patients was published. The study analysed the data of 734 European patients with EGPA and revealed that different baseline characteristics were present among MPO-ANCA, PR3-ANCA, and ANCA-negative groups [
10]. Given the ethnic and geographical differences in patients with AAV between Asia and Europe, it may be meaningful to evaluate the clinical features of Korean patients with EGPA according to the presence and absence of PR3-ANCA. Hence, in this study, we investigated the clinical implications of PR3-ANCA in Korean patients with EGPA.
DISCUSSION
In this study, we analysed the differences in clinical features at diagnosis and prognoses during follow-up between EGPA patients with PR3-ANCA and those without PR3-ANCA. We first compared the major clinical characteristics between EGPA patients that were included in our study and those included in previous studies [
6,
10]. Age at diagnosis (54 years vs. 40–60 years), pulmonary involvement (65.3% vs. 34%–76%), heart involvement (18.4% vs. 12%–49%), kidney involvement (26.5% vs. 12%–49%), and peripheral nerve involvement (59.2% vs. 43–84%) did not differ between the two groups. However, EGPA patients included in our study had higher rates of PR3-ANCA positivity (12.2% vs. 0%–3.2%) and ear, nose, and throat involvement (81.6% vs. 53%–78%) than those in previous studies. However, skin involvement was less often observed in EGPA patients that were included in our study compared to those in previous studies [
6,
10].
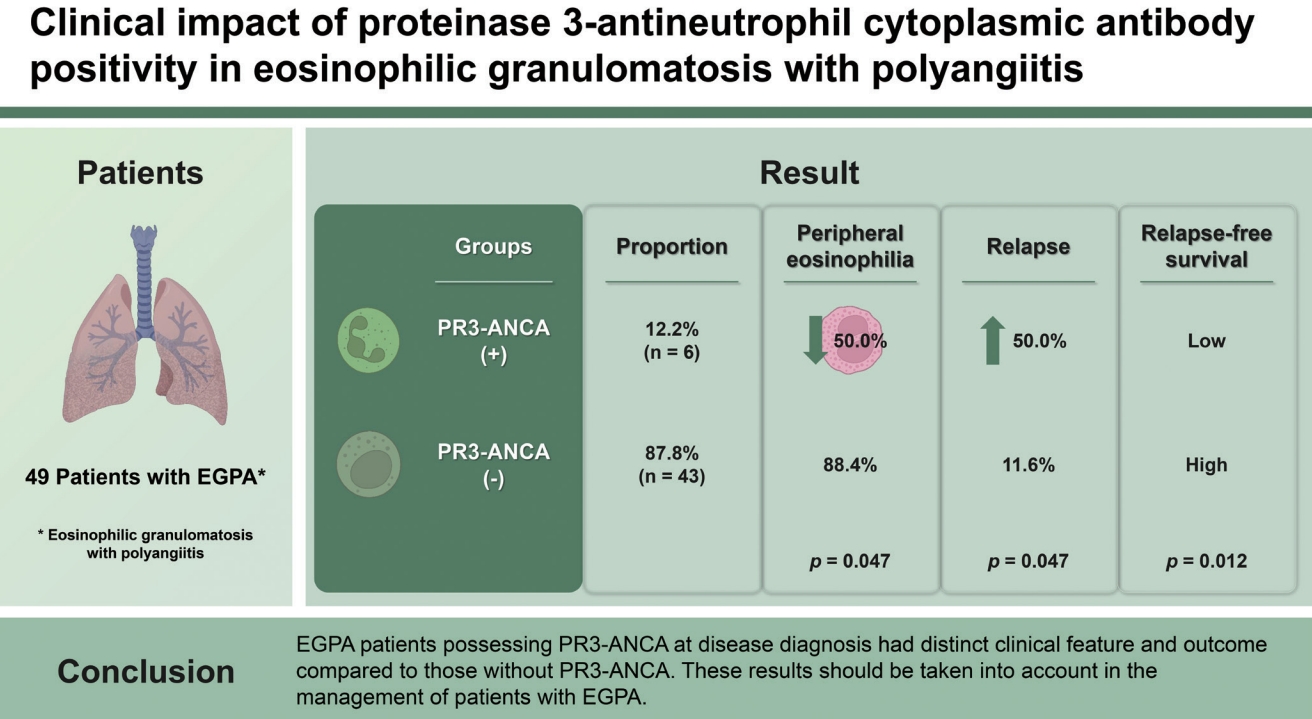
Our results demonstrated that patients with PR3-ANCA exhibited less frequent peripheral eosinophilia, while disease relapse was more common. Importantly, differences and similarities were found between our study and a previous publication by Papo et al. [
10]. First, the report by Papo et al. [
10] showed that EGPA patients with PR3-ANCA had active asthma and peripheral neuropathy less often than those without PR3-ANCA. Meanwhile, it has been shown that EGPA patients that had PR3-ANCA had cutaneous manifestations, pulmonary nodules, and reduced eosinophil counts more frequently than those without PR3-ANCA [
10]. One important finding was that reduced eosinophil count was more common in EGPA patients with PR3-ANCA, and this was demonstrated in our study; however, there was no significant difference in absolute eosinophil count between the two groups. The frequencies of peripheral neuropathy and cutaneous manifestations did not differ in the present study, and pulmonary nodules were not found in our study population. A previous study demonstrated that EGPA patients with PR3-ANCA and MPO-ANCA showed lower cumulative relapse-free survival rates than those in the ANCA-negative group [
10]. In line with this observation, we also found that EGPA patients with PR3-ANCA exhibited a lower cumulative relapse-free survival rate than those without PR3-ANCA (
p = 0.012), but not in ESRD-free survival rate.
PR3-ANCA is generally regarded as a potential risk factor for relapse in AAV patients [
8,
17]. In particular, as demonstrated in a study by Fussner et al. [
18], the increase in PR3-ANCA levels was closely related to the forthcoming relapse in AAV, and relapses occurred within 1 year after the elevation of PR3-ANCA titres. Nonetheless, it is still uncertain whether the presence of PR3-ANCA is predictive of relapse in patients with EGPA. However, in line with the results of the study of Papo et al. [
10] and the results of our study, PR3-ANCA appears to potentially increase the risk of relapse in EGPA patients. Therefore, we carefully suggest that physicians should treat EGPA patients with PR3-ANCA, considering the possibility of relapse.
A previous study conducted by Papo et al. [
10] included four EGPA patients with PR3-ANCA who had pulmonary nodules. Based on the 2007 EMA algorithm for the classification of AAV, if pulmonary nodules had been considered as one of the surrogate markers of granulomatosis with polyangiitis (GPA), such as fixed, nodular and cavitary lesions in the lower respiratory tract, these four EGPA patients with pulmonary nodules should have been classified as GPA [
5]. According to the 2007 EMA algorithm, patients who did not undergo biopsy, can be classified as having GPA if they have both surrogate markers of GPA and ANCA positivity [
5]. Furthermore, according to the ACR/European Alliance of Associations for Rheumatology (EULAR) 2017 provisional classification criteria for GPA, if they had sino-nasal symptoms such as sinusitis, they could be preferentially classified as having GPA (≥ 5 points) based on sino-nasal symptoms (3 points), PR3-ANCA positivity (5 points), pulmonary nodule (2 points), and peripheral eosinophilia (−3 points) [
19].
Given this ambiguity in differentiating EGPA patients that have PR3-ANCA from GPA patients with PR3-ANCA, we wondered whether the clinical features of the two groups might differ (
Supplementary Fig. 1). Of the 242 patients, we selected 29 GPA patients that had PR3-ANCA and compared their clinical features with six EGPA patients with PR3-ANCA. GPA patients with PR3-ANCA had the clinical features that were similar to those of EGPA patients with PR3-ANCA except for cutaneous and gastrointestinal manifestations that occurred more frequently in EGPA patients with PR3-ANCA than GPA patients with PR3-ANCA (
Supplementary Table 1). In addition, there was no significant difference in the cumulative relapse-free survival rates between the two groups (
Supplementary Fig. 2).
At the entry of the present study, we intended to investigate the differences in variables among PR3-ANCA positive, MPO-ANCA positive and ANCA-negative groups of EGPA patients. However, in the Kaplan-Meier analysis, the occurrence of relapse in EGPA patients was apparent in the PR3-ANCA group compared to the MPO-ANCA positive and ANCA-negative groups; furthermore, no difference in the cumulative relapse-free survival rates was found between EGPA patients with MPO-ANCA and those without any type of ANCAs. Meanwhile, EGPA patients with PR3-ANCA exhibited a significantly lower cumulative relapse-free survival rate than those without any type of ANCA. They also tended to show a lower cumulative relapse-free survival rate than those with MPO-ANCA even though not statistically significant (
Supplementary Fig. 3). On the other hand, the main purpose of this study was to clarify the clinical significance of PR3-ANCA as a risk factor for relapse in EGPA patients. Therefore, we divided EGPA patients into two groups according to PR3-ANCA positivity, and we investigated the differences between EGPA patients with PR3-ANCA and those without PR3-ANCA.
Although the advantage of this study is that this was the first study to evaluate the features and outcomes of Korean patients with EGPA according to PR3-ANCA status, the small number of EGPA patients with PR3-ANCA and the retrospective study design should be clearly addressed as a critical limitation. Additionally, serial results of PR3-ANCA might have highlighted the clinical implication of PR3-ANCA in three EGPA patients who had PR3-ANCA but not relapse. However, since serial PR3-ANCA results during follow-up were not available in two of three EGPA patients with PR3-ANCA but without relapse, we could not analyse the significance of the follow-up result how PR3-ANCA affected EGPA relapse.
In conclusion, we showed that EGPA patients that had PR3-ANCA at diagnosis have distinct clinical features and outcomes compared to those without PR3-ANCA. Therefore, we believe that these results should be considered when managing EGPA patients.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print



