HLA-DR Antigen Expression on the Thyrocytes of Graves’ Disease Patients
Article information
Abstract
To confirm the expression of HLA-DR antigen on the thyrocytes of Graves’ disease patients and study the relation between the degree of DR antigen expression and clinical or laboratory indices, double immunofluorescence and immunoenzymatic staining were performed on frozen thyroid sections. DR antigen was expressed on the thyrocytes of 8 of 11 patients with Graves’ disease (73%) but not found on the thyrocytes from 6 normal controls. The degree of DR antigen expression had no apparent correlation with the age, duration of the disease, activity of TBII, titers of antimicrosomal and antithyroglobulin antibody. There was no apparent spatial or quantitative relation between lymphoid follicle formation and the DR antigen expression on the thyroid follicles. But DR antigen tended to be expressed on the thyroid tissue with interstitial, lymphocyte infiltration. In conclusion, DR antigen was expressed on the thyrocytes of Graves’ disease patients but the clinical and immunological significance remains to be clarified.
INTRODUCTION
Graves’ disease has been regarded as an organ-specific autoimmune disease due to the autoantibodies directed to the receptors of Thyroid Stimulating Hormone (TSH) or their adjacent antigenic domains.1–4) However, the immunological mechanisms leading to the development of these autoantibodies are still elusive, albeit many theories presented so far.5–13)
Recently, it was reported that the HLA-DR antigen was expressed on the thyrocytes of the patients with Graves’ disease and Hashimoto’s disease.14, 15) Moreover, the DR antigen was reportedly able to be induced on the normal thyrocytes by lectins.16) These findings suggest that the thytrocytes might become antigen-presenting cells in an appropriate condition to induce or amplify the autoimmune processes,17) considering that the DR antigen is necessary for antigen presentation.18–20) DR antigen was also found to be expressed on the islet cells of the patients with type I diabetes mellitues21) and the bile duct cells of the patients with primary biliary cirrhosis,22) suggesting the possible role of the aberrant DR antigen expression in the broad gamut of autoimmune diseases. However, the immunological impact of these phenomena is not clarified and it is still controversial whether lymphocyte infiltration in the thyroid gland is related to the DR antigen expression on the thyrocytes or not.
We conducted this investigation to document the HLA-DR antigen expression on the thyrocytes of Graves’ disease patients and to study the relationship between the degree of DR antigen expression, & clinical or laboratory indices, and the degree of lymphocyte infiltration in the thyroid gland.
MATERIALS AND METHODS
1. Materials
Specimens from the Graves’ disease patients were obtained during thyroidectomy from 11 patients who were in euthyroidism with the antithyroid drug and those of normal controls, from the adjacent normal portion of 6 patients with nodular goiter. The specimens were immediately frozen below −20°C and thawed within 1 month of storage to be cut into 6 μm sections for double indirect immunofluorescence staining.
Monoclonal antibody to human HLA-DR antigen was produced at the Department of Microbiology, College of Medicine, Seoul National University23) (1BD9-2). Sera containing antimicrosomal antibody were obtained from 2 patients with Graves’ disease whose titer of antimicrosomal antibody was over 1:12802 without detectable antithyroglobulin antibody. FITC-labeled or unlabeled rabbit antimurine IgG (heavy & light chain specific), and rhodamine-labeled rabbit antihuman IgG (heavy & light chain specific) were purchased from Cappel (Malvern, PA, USA). Monoclonal anti-CD5, CD4, CD8 antibodies (T1, T4, T8 antibody, respectively) and AP-AAP complex were purchased from DAKO Corp. (Santa Barbara, CA, USA). Bovine serum albumin, Fast Red TR Salt (5-chloro-2-toluidinediazonium chloride hemizine chloride), levamisole and Naphthol AS-MX sodium salt (3-hydroxy-2-Naphthoic acid 2,4-dimethylanilide) were purchased from Sigma (St. Louise, MO, USA).
METHODS
1. Immunofluorescence Staining
The appropriate cut sections of frozen tissue were incubated for 30 min in a humid chamber at 37°C with the culture supernatant of the 1BD9-2 cell line and a 1:100 dilution of the sera containing antimicrosomal antibody. Then the sections were washed 3 times with phosphate buffered saline for a total of 30 min, and were reincubated with a 1:20 dilution of FITC-labeled rabbit antimurine IgG and rhodamine-labelled rabbit antihuman IgG in a phosphate buffered saline containing 0.1 % bovine serum albumin for 30 min in a humid chamber at 37°C. The sections were washed 3 times with phosphate buffered saline again for a total of 30 min, and examined under a fluorescent microscope (American Optical) after mounting with glycerol in phosphate buffered saline. The negative control slide was made by incubating the adjacent cut sections with the second antibodies only. Autofluorescence was excluded by scrutinizing unstained cut sections under the fluorescent microscope, and the cross-immunoreactivities between human IgG & antimurine IgG, or murine IgG & antihuman IgG were tested by cross-staining of the cut sections to yield negative results. The number of prominently bright-green follicles compared to the negative control slide was counted in over 50 thyroid follicles and the ratio was rounded to one decimal.
2. Alkaline Phosphatase-Antialkaline Phosphatase Staining
The specimen of one patient which showed large clusters of DR antigen positive cells in the vicinity of the thyroid follicles with double immunofluorescence staining was subjected to AP-AAP (Alkaline Phosphatase-Antialkaline Phosphatase) double bridge staining24,25) using T1, T4 or T8 monoclonal antibody as the first antibody. The cut frozen sections were incubated with a 1:40 dilution of the first antibody in phosphate buffered saline for 30 min at room temperature and washed with phosphate buffered saline 3 times for a total of 6 min subsequently. Then they were incubated with a 1:50 dilution of antimurine IgG for 30 min at room temperature and washed again 3 times with phosphate buffered saline for a total of 6 min followed by incubation with 1:50 dilution of AP-AAP complex and rewashing. The incubation with the second antibody and the AP-AAP complex were recapitulated to make a “double bridge” and the processed sections were incubated with a color substrate solution made by dissolving 10 mg of Fast Red TR Salt, 2.4 mg of levamisole and 2 mg of Naphthol AS-MX sodium salt in 0.1 M Tris buffer (pH 8.2), 10 ml. The final sections were counterstained with hematoxylin.
3. Measurement of the Autoantibodies & TSH Receptor Antibody
The titers of antimicrosomal antibody and antithyroglobulin antibody were measured with the hemagglutination method using the kits produced by Fujirebio (Japan). The activity of TBII (TSH Binding Inhibitory Immunoglobulin) was measured using the kits produced by R.S.R. Limited (Cardiff, Wales, UK).
4. Scrutiny of Lymphocyte Infiltration
Ten fields of hematoxylin-eosin stained sections were examined in low-power microscopy to grade the degree of lymphoid follicle formation from − to + + + +. The presence of interstitial lymphocyte infiltration was searched apart from lymphoid follicle formation.
5. Statistical Analysis
Kendall’s W test of concordance was employed to study the correlation between the degree of DR antigen expression on the thyrocytes, and clinical or laboratory indices, the degree of lymphocyte infiltration in the thyroid gland.
RESULTS
1. DR Antigen Expression on the Thyroid Tissue
All of the thyroid follicles of both the normal controls and the patients with Graves’ disease were stained with rhodamine suggesting the presence of microsomal antigen on the thyrocytes (Fig. 1).

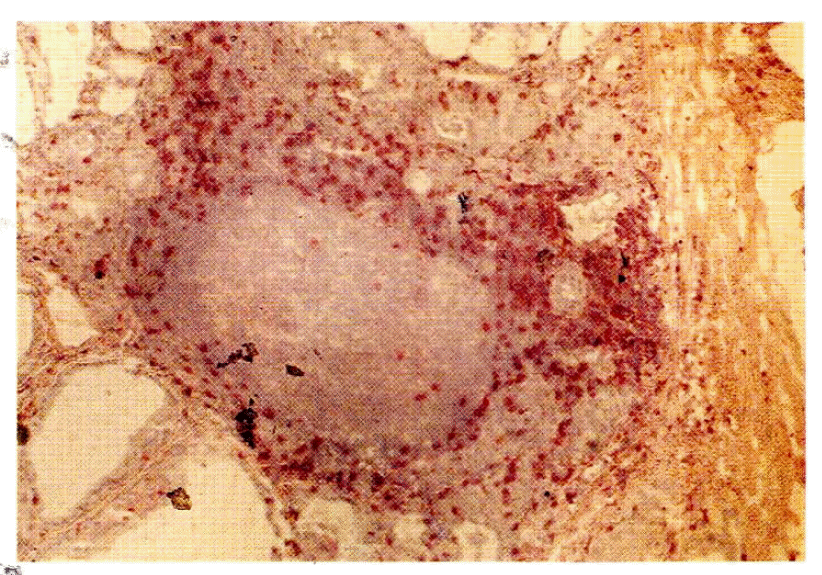
Closed arrow indicates positive rhodamine staining on the thyroid follicles of Graves’ disease patient. A large patch of cells adjacent to the thyroid follicles shows negative rhodamine staining (long arrow), but the central area of the patch shows positive rhodamine staining (open arrow).
DR antigen was not found at all on the frozen sections of the normal thyroid tissues from 6 subjects. Strong or weak autofluorescence that could be discerned from true staining by the yellow color instead of green, was observed occasionally along the inner side of large or small vasculatures (Fig. 2).
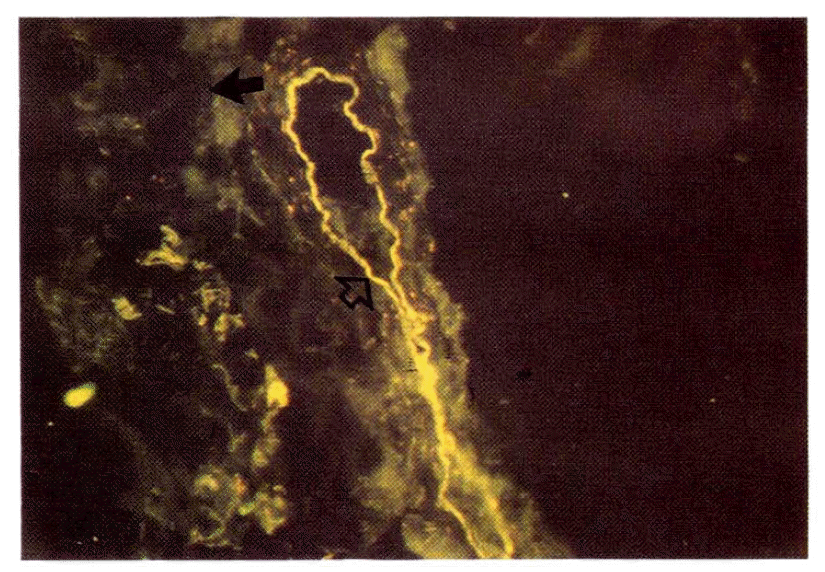
The thyroid follicles of the normal control were DR-antigen negative (closed arrow). The yellow line along the inner side of an arteriole represents autofluorescence (open arrow) (See text.)
Of 11 patients with Graves’ disease, 8 patients (73%) showed DR antigen expression on their thyroid follicles, and the ratio of DR antigen-positive follicles varied between 10–80% (Fig. 3, Table 1). The staining pattern was patch, i.e. DR-positive thyrocytes were rather aggregated. There was no apparent correlation between the degree of HLA-DR antigen expression on the thyroid follicles and the clinical or laboratory indices including age, duration of the disease, the activity of TBII, and the titers of antimicrosomal antibody & antithyroglobulin antibody (p>0.1 in all tests).

The thyrocytes of Graves’ disease patients were DR antigen-positive (short arrow), as is the large patch of cells adjacent to the thyroid follicles (long arrow).
2. Nature of Infiltrating Cells around Thyroid Follicles
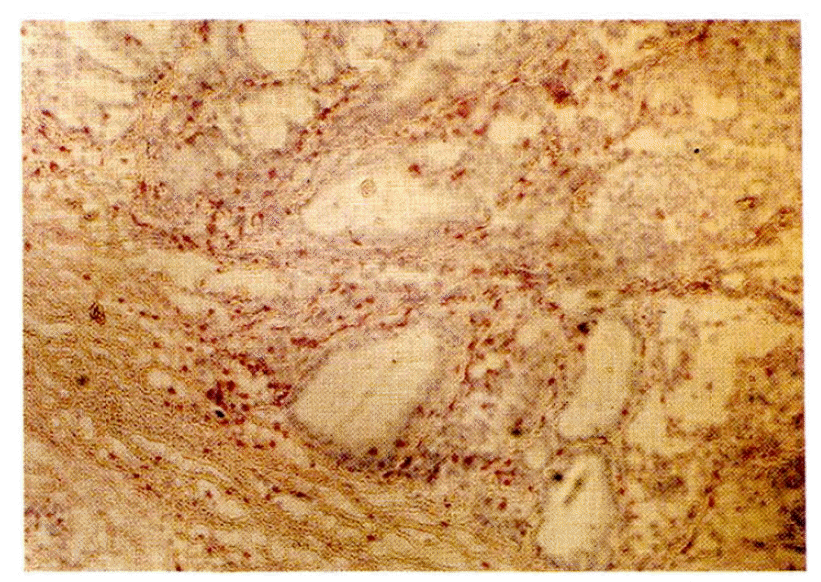
On the several frozen sections from the patients with Graves’ disease, large or small clusters of microsomal antigen-negative, DR antigen-positive cells were observed after double immunofluorescence staining (Fig. 1, 3). The cells in those clusters were positively stained with the T1 antibody except in the central area (Fig. 4). The lymphocytes stained with T1 antibody also stained with T4 antibody or T8 antibody in the staining of the adjacent sections of the tissue (Fig. 5, 6). There was no apparent discrepancy in the distribution patterns of T4-positive helper/inducer T-lymphocytes and T8 positive suppressor/cytotoxic T-lymphocytes but the quantitative ratio was about 3:1. The lymphocytes of both subsets were also scattered in the thyroid follicles (Fig. 7). Meanwhile, the cells in the central area of those DR antigen-positive clusters showed positive rhodamine staining not only in case of the regular double immunofluorescence staining but also in the control staining, i.e. in the absence of the antimicrosomal antibody, suggesting the presence of a surface immunoglobulin on the cells in the central area (Fig. 1, 3). So the cells in the central area of those clusters were thought of as B lymphocytes considering that they had a surface immunoglobulin in addition to the DR antigen and were not stained with T1 antibody (Fig. 4). Germinal centers were also observed occasionally in the central area of those DR-positive clusters with light microscopy.
There was no apparent spatial or quantitative relation between the lymphoid follicle formed and the DR antigen expression on the thyroid follicles (p>0.1). But the degree of lymphoid follicle formation and the presence of interstitial lymphocyte infiltration did not coincide, and all 4 cases with interstitial lymphocyte infiltration showed DR antigen expression on 50% or above of their thyroid follicles examined (Table 1).
DISCUSSION
The negative DR antigen staining of the normal thyroid follicle is consistent with the previous reports of other investigators and the classical theory that the class II antigens are localized on the surface of immune cells including B lymphocytes, activated T-lymphocytes and various bone marrow-derived antigen presenting cells such as the macrophage or dendritic celll.18,19,26,27) Meanwhile, there are occasional reports that HLA-DR antigen was found on the endothelial cells of normal kidney glomeruli,28) and Hanafusa et al. reported the presence of a few DR antigen positive endothelial cells on the sections of normal thyroid tissue. We observed linear or caret-like fluorescence along the inner side of large or small vessels, respectively, but the color was rather yellow and fluorescence was also found on the unstained frozen sections indicating that it was due to autofluorescence. The autofluorescence along the inner side of the vasculatures may be accounted for by the presence of elastic fiber or type IV collagen.29)
The observation that DR antigen was found on the thyroid follicles of patients with Graves’ disease is compatible with the results of other investigators.14,15) The presence of 3 patients whose frozen sections gave negative results with DR staining and the variable degree of DR antigen expression in the positive cases suggest the possibility that DR antigen expression may not be a crucial event in the pathogenesis of Graves’ disease or DR antigen may be expressed on the thyrocytes only in a certain period of the disease. It is also probable that the degree of DR antigen expression may be related to the HLA type of patients considering the reports that the incidence of Graves’ disease is related to the HLA type.12,13) But it must to borne in mind in the interpretation of these results that the cut section represents only a certain 2-dimensional space of the whole thyroid gland.
The finding that the degree of DR antigen expression on the thyroid follicles has no relation with the clinical and laboratory indices can also be explained in the same manner as the variable degree of DR antigen expression itself.
The immunological significance of aberrant DR antigen expression on the epithelial cells such as thyrocytes is far from clear, although it was elucidated that induction of DR antigen expression on the cultured normal thyrocytes is, at least partly, due to gamma-interferon secreted by T-lymphocytes.30,31) The epithelial cells which have surface DR antigen from any cause might present their other normal or abnormal surface antigens to the helper/inducer T-lymphocytes simulating antigen presenting cells if other conditions for the antigen presentation are permissive,16,17) considering that the DR antigen on the antigen presenting cells is co-presented to the autologous helper/inducer T-lymphocytes with foreign antigens.20) But it may merely be an epiphenomenon without specific consequence reflecting a response to various nonspecific or nonimmunological stimuli, or have a protective effect against those stimuli.32) Recently, it has been reported that the thyrocytes and endothelial cells with aberrant DR antigens on their surface could present autologous or heterologous antigens to the helper T-lymphocytes.33,34) But there seems to be no direct evidence that DR antigen expression on the epithelial cells plays a role in the induction or amplification of the autoimmune processes.
The clusters of DR antigen-positive, microsomal antigen-negative cells seemed to be lymphocytes judging from the light microscopic findings, which was confirmed later in one case by immunoenzymatic staining. The positive rhodamine staining on the cells in the central area of the large clusters was an unexpected event, but the same positive staining in the control slides gave an answer to this question. The quantitative T4/T8 ratio in that case was similar to the results of other studies concerning lymphocyte infiltration in Graves’ disease.15) DR antigen-positivity of the infiltrating T-lymphocytes suggest that they were “activated.” 35,36) The infiltrating T-lymphocytes might be a probable candidate for a source of gamma-interferon which can induce the thyrocytes to express DR antigen. The present finding that all 4 cases with diffuse interstitial lymphocyte infiltration expressed DR antigen on over 50%, of their thyroid follicles supports the suggestion that infiltrating lymphocytes might be responsible for DR antigen expression on the thyroid follicles. But in contrast to the interstitial lymphocyte, the lymphoid follicles formed in the thyroid tissue did not seem to be directly related with the DR antigen expression on the thyrocytes.
Acknowledgements
The authors are indebted to Wang Jae Lee, M.D. and Prof. Ka Yong Jang, Dept. of Anatomy, College of Medicine, Seoul National University for their technical & material aid in performing AP-AAP staining.