Clinical Significance of On-Treatment Triglyceride Level in Patients Treated by Percutaneous Coronary Intervention for Non-ST-Segment Elevation Acute Coronary Syndrome
Article information
Abstract
Background/Aims
The use of statins in patients with acute coronary syndrome (ACS) has increased, and reduced levels of low-density lipoprotein cholesterol (LDL-C) lead to lower coronary event rates. We studied the effect of lipid levels during statin treatment on prognosis in patients with ACS and percutaneous coronary intervention (PCI).
Methods
Between January 2005 and May 2007, 325 ACS patients who underwent PCI and received statins were evaluated. We measured serum lipid levels at baseline and 4 weeks. The relationships between on-treatment levels of triglyceride (TG) and LDL-C and one-year major adverse cardiac events (MACE) were assessed.
Results
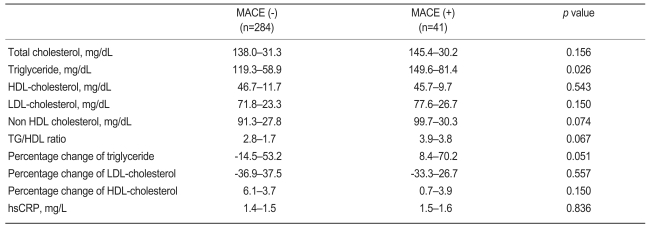
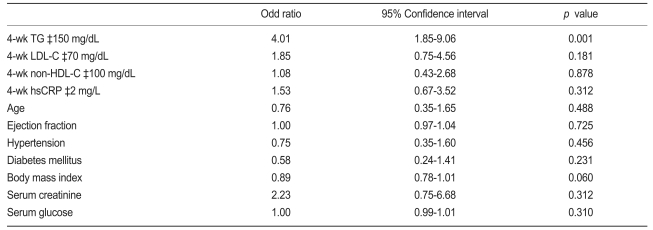
At 4 weeks, the mean LDL-C level was 72.5±23.8 mg/dL and the mean TG was 123.2±62.8 mg/dL. MACE occurred in 41 cases (12.6%). Baseline serum lipid levels were similar between the patients with and those without MACE. However, the patients with MACE showed significantly higher TG level at 4 weeks (149.6±81.4 vs. 119.3±58.9 mg/dL, p=0.026) than those without. High on-treatment TG level (≥150 mg/dL) were associated with increased adverse events compared to lower TG level in a univariate analysis (hazard ratio [HR], 3.3; p<0.001). In a multivariate analysis, high 4-week TG level after statin treatment was an independent predictor for MACE (HR, 4.01; 95% confidence interval, 1.85 to 9.06; p=0.001), however, baseline TG and LDL-C levels were not.
Conclusions
High on-treatment TG level (≥150 mg/dL) was associated with a higher risk of MACE. This finding supports the concept that achieving low TG levels may be an important therapeutic parameter in statin-treated patients following ACS and PCI.
INTRODUCTION
One of the targets most emphasized in risk reduction for cardiovascular diseases is lowdensity lipoprotein cholesterol (LDL-C). Recent large-scale lipid-lowering trials have suggested that hydroxylmethyglutaryl-CoA reductase inhibitors (statins) provide benefits in the primary and secondary prevention of coronary artery disease [1-4].
The benefits of statin treatment are mediated through reducing LDL-C levels. A metaanalysis showed that lowering LDL-C below 57 mg/dL can theoretically reduce the rate of new coronary heart disease (CHD) event to zero, and that lowering it below 67 mg/dL stopped the progression of atherosclerosis [5]. Additionally, the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) trial in patients with acute coronary syndrome (ACS) showed that high-dose statin therapy, with a median LDL-C level of 62 mg/dL, was better than the standard therapy [6]. Thus, the 'lower is better' hypothesis is currently accepted, and the National Cholesterol Education Program-Adult Treatment Panel-III (NCEP-ATP-III) guidelines also recommend that target LDL-C be lower than 70 mg/dL in very high-risk patients, including ACS patients [7].
Many clinical trials of statins have demonstrated significant reductions in CHD events, compared with placebo [8]. In general, however, a significant risk of coronary events remains, so additional treatment methods are necessary to further reduce the risk.
Analyses of patients who received statins but still had CHD events may indicate which factors are important for additional risk reduction. Lipid-lowering trials have shown that the lowest lipid levels after statin treatment were achieved in the first month, and then the lowered lipid level remained stable during the study period [6]. As a result, examination of lipid levels at one month and analysis of its relationship with the CHD events may provide information about whether LDL-C should be further reduced or whether other targets should be addressed.
We examined the on-treatment lipid levels at 4 weeks in patients with ACS who received percutaneous coronary intervention (PCI) in an attempt to understand the relationship with prognosis.
METHODS
Subjects and protocol
Between January 2005 and May 2007, 350 consecutive ACS patients who underwent PCI and received statins before discharge were evaluated. Exclusion criteria included ST-segment-elevation myocardial infarction (MI), including true posterior MI, failed PCI, in-hospital MI, or repeated revascularization, allergy to statins, administration of fibrates or fish oil, known inflammatory, neoplastic, or infectious disease, and PCI within the previous 12 months.
PCI was performed according to current clinical practice at the physician's discretion. Angiographic success of PCI was defined as TIMI III flow, with residual stenosis below 20%. All patients involved in the study had undergone successful procedures. After the procedure, the patients received 75 mg of clopidogrel for 12 months and aspirin indefinitely. Statins were dosed as soon as possible after hospitalization, regardless of baseline lipid level, and prior to discharge, at the latest. Angiotensin-converting enzyme inhibitors and beta blockers were also prescribed at the physician's discretion.
Toal cholesterol, triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C) were measured by an automated chemial analyzer (Roche diagnostics, Mannheim, Baden-Wurttemberg, Germany) after a 12 hour overnaight fat. LDL-C was measured directly. Lipid and lipoprotein levels were obtained at baseline, 4 weeks, 6 months, and 12 months.
The endpoint was defined as the occurrence of a major adverse cardiac event (MACE), including cardiac death, non-fatal MI, and any coronary repeated revascularization (surgery or PCI). Only the first such event was counted as the end point. Twelve months follow-up data were obtained in a total of 325 patients; in 19 cases, statins were stopped, and six cases of MACE occurred before 4 weeks, as determined by inspection of medical records or direct contact with the patients.
All patients gave informed consent according to a protocol approved by the institutional review board of the hospital.
Statistical analysis
All measurements are presented as mean±standard deviation. Inter-group analysis was conducted using the independent t-test, χ2 test, or Fisher's exact test, using the SPSS version 11.0 (SPSS Inc., Chicago, IL, USA). The ANOVA test and Games-Howell test were performed to compare statin groups. The Kaplan-Meier method was used for cumulative MACE free survival analysis. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated using selected cut-off points as references. Cut-off points were derived from the NCEP-ATP-III guidelines [7]: that is, LDL-C <70 mg/dL and triglycerides <150 mg/dL. Additionally, other selected cut-off points for non-HDL-C <100 mg/dL and C-reactive protein (CRP) <2 mg/L were evaluated [7,9,10]. The effect of covariates on survival time was tested by Cox proportional-hazards regression analysis. Statistical significance was set at p<0.05.
RESULTS
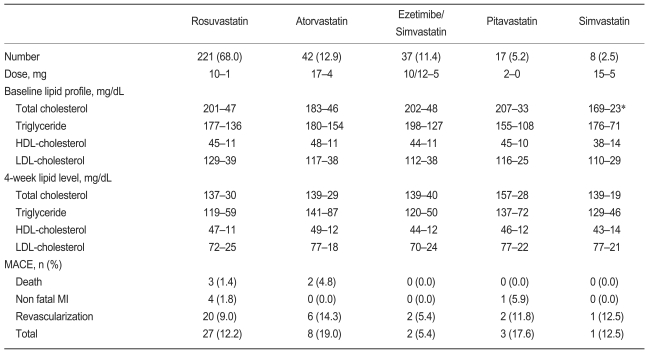
A total of 325 patients were identified by the study criteria. Of these, 68.0% received rosuvastatin, 12.9% atorvastatin, 11.4% ezetimibe/simvastatin, 5.2% pitavastatin, and 2.5% simvastatin. During 12 months of follow-up, MACE occurred in 41 patients (12.6%) including five cases of cardiac death, five cases of non-fatal MI, and 31 cases of repeated revascularization.
Baseline clinical characteristics and procedural variables according to the occurrence of MACE are shown in Table 1. No significant difference was found in the most relevant clinical characteristics between the two groups. We used drug-eluting stents in most cases (98.5%), and over 33% of the patients received multi-vessel stenting. All angiographic and procedural characteristics were similar between the groups.
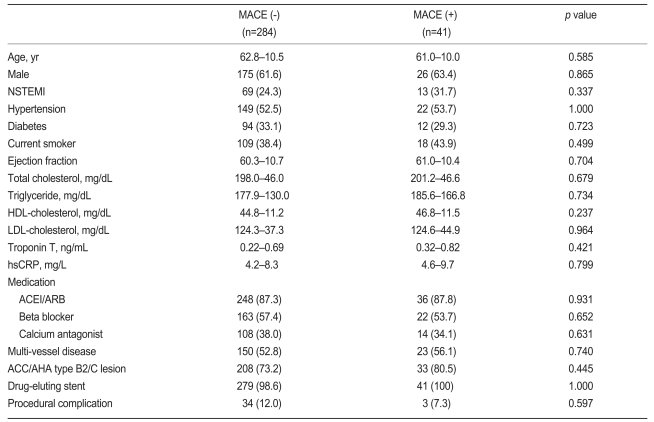
Baseline characteristics of subjects according to absence or presence of major adverse cardiac events
At 4 weeks, the mean LDL-C level was 72.5±23.8 mg/dL and the mean TG was 123.2±62.8 mg/dL. Of the patients, 49.8% had LDL-C ≥70 mg/dL, and 24.3% had TG ≥150 mg/dL. The patients with MACE had significantly higher on-treatment TG level than did those without (149.6±81.4 vs. 119.3±58.9 mg/dL, p=0.026, Table 2). Low on-treatment LDL-C (<70 mg/dL) was not associated with MACE compared to higher LDLC in a univariate analysis. Rather, fewer MACE were observed in patients with low on-treatment TG level (HR, 3.3; 95% CI, 1.8 to 6.0; p<0.001) and low on-treatment non HDL-C (HR, 2.1; 95% CI, 1.1 to 3.8; p=0.019) compared to those with higher levels of TG and non-HDL-C (Fig. 1).
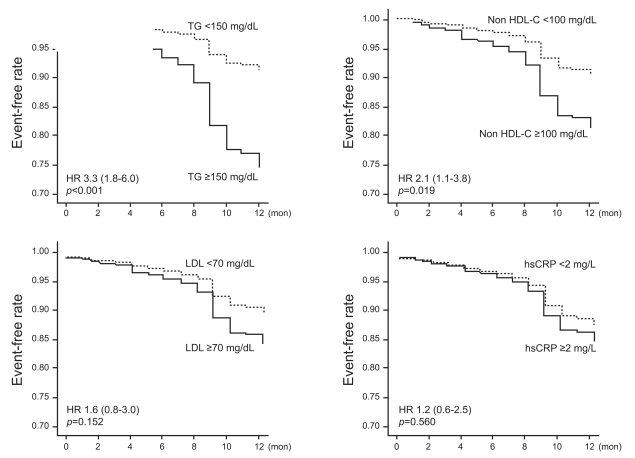
One-year event-free rate according to on-treatment 4-week lipid levels.
TG, triglyceride; HDL, high density lipoprotein; LDL, low density lipoprotein; hsCRP, high sensitivity C-reactive protein.
Lipid levels and clinical outcomes according to the statin used are shown in Table 3. Most patients were treated with standard doses of statins. No significant difference in 4-week lipid levels and MACE were found according to the type of statin regimen. Multivariate analysis revealed that on-treatment TG level was the only significant independent predictor of MACE (Odd Ratio, 4.09; 95% CI, 1.85 to 9.06; p=0.001; Table 4). Baseline lipid levels were not associated with MACE in patients treated with statins.
DISCUSSION
This study demonstrates that on-treatment TG ≥150 mg/dL was associated with a higher risk of MACE. This supports the concept that TG levels are an important target in patients who undergo statin treatment.
LDL-C is a well-established risk factor for CHD. Observational studies have consistently shown a positive relationship between CHD risk and LDL-C levels [8,11,12]. Trials in both primary prevention and secondary prevention settings have shown that the risk of suffering a CHD event during the course of the study was closely related to ontreatment LDL [1-4,13]. Thus, the NCEP-ATP-III guidelines also recommend that the target LDL-C be lower than 70 mg/dL in very high-risk patients, including ACS patients [7]. However, lipid-lowering therapy using statins led to a relative risk reduction of only 25-30% in most large randomized controlled trials [8]. Thus, factors other than LDL-C may be important: the recently published INTERHEART study emphasized dyslipidemia as an important risk factor, and the NCEP-ATP-III considers non-HDL-C as a secondary target [14].
Our findings emphasize that lowering TG is necessary, beyond lowering LDL-C, in patients treated with statins. A meta-analysis of observational studies of TG and CHD events showed that an 89 mg/dL increase in TG level had an independent risk prediction rate of 14% in men and 37% in women [15]. A recent meta-analysis of 29 published reports showed about a 70% increase in primary CHD events in men and women in the highest, compared with the lowest, tertile of the TG distribution, even after adjusting for age, gender, smoking, and lipid measurements [16]. The importance of on-treatment TG has also been demonstrated in other studies. A large prospective Chinese study found TG to be predictive of CHD mortality, even in a setting of low total cholesterol [17]. In analyses of the PROVE IT-TIMI 22 trial, low on-treatment TG (<150 mg/dL) was associated with reduced CHD risk compared with higher TG after adjustment for LDL-C [18].
Some reports have shown that TG is an important factor in atherosclerosis. In a hypertriglyceridemic state, the accumulation of remnants results in a proinflammatory and oxidative milieu that may enhance the expression of adhesion molecules, foam cell formation, and smooth muscle cell toxicity [19]. High TG levels cause increases in small dense LDL, the substance that most readily causes atheroclerosis, and also reduce HDL, thereby accelerating atherosclerosis [20]. The correlation between plasma TG and atherogenic remnants is coupled with the excess risk of CHD with combined elevations of LDL-C and TG [17]. Thus, a strategy combining lowering of both LDL-C and TG, rather than intensive LDL-C-lowering therapy alone, would be more effective for the treatment of patients with ACS.
LDL-C, HDL-C, and CRP were less important in the present study, which may have been due to the limitations of this small observational study or might be characteristic of patients already treated with statins. All patients received powerful statin treatment, so their average lipid profile was well controlled and below target levels; this may have resulted in the relatively lower importance of other risk factors. However, this study should not be understood to detract from the importance of those risk factors. Rather, it is desirable to understand the results of this research as emphasizing the powerful control of TG in addition to the traditional risk factors.
Limitations of this study include: 1) weak statistical power due to the small patient group and the low event rate; 2) the original purpose of this research was not to prove the hypothesis that combined low on-treatment LDL and TG is better than low ontreatment LDL-C; 3) because the statin regimens used in the patients were not same, treatment agent may be another confounding variable; and 4) the failure in presenting the target levels of on treatment TG. Our findings obviously need to be complemented by future large-scale randomized trials.
In conclusion, on-treatment TG ≥150 mg/dL was associated with a higher risk of MACE. This finding supports the concept that achieving low TG may be an important therapeutic parameter in statin-treated patients following ACS and PCI.