A case of papillary fibroelastoma in the left ventricle
Article information
Abstract
Cardiac papillary fibroelastoma (CPF) is a rare and benign primary cardiac neoplasm of unknown prevalence. The incidence of CPF in the left ventricle is lower than that in other parts of the heart. A 65-year-old female was referred to our cardiology department for evaluation of a cardiac mass of the left ventricle. Transthoracic echocardiography revealed a 1.8 × 1.7 cm highly mobile round mass attached by a stalk to the apical inferior wall of the left ventricle with an echolucent area. The mass was successfully removed without any postoperative complications and was identified as a CPF.
INTRODUCTION
The incidence of primary cardiac tumors is low, at 0.001% to 0.03% [1-3]. The advent of variable cardiac imaging modalities, including echocardiography and multidetector computed tomography (CT), has led to the identification of an increasing number of cases of asymptomatic cardiac tumors. Cardiac papillary fibroelastoma (CPF) is a rare and benign cardiac tumor that can arise anywhere in the heart, but usually involves cardiac valves. The aortic valve is most commonly involved [4]. Moreover, the incidence of CPF in the left ventricle is lower than that in other parts of the heart [3]. Here we report a case of left ventricular CPF detected by echocardiography and successfully surgically resected.
CASE REPORT
A 65-year-old woman was referred to our cardiology department for evaluation of a cardiac mass of the left ventricle. Prior to the referral, she was admitted to another hospital with a complaint of chest pain of 3 days' duration. Coronary angiography and echocardiography were performed before she visited our hospital. Coronary angiography showed no significant coronary disease, but a mass was found by echocardiography. She had been treated for hypertension and a stroke she suffered 5 years prior.
On physical examination, her blood pressure was 110/70 mmHg, and her pulse rate was 62 beats/min. Her heart sounds were normal and no murmur was heard. The electrocardiogram showed normal sinus rhythm without abnormal findings. Transthoracic echocardiography revealed a 1.8 × 1.7 cm highly mobile round mass attached by a stalk on the apical inferior wall of the left ventricle with an echolucent area (Fig. 1A). The mass was easily compressed (Fig. 1B) and returned to its original shape (Fig. 1C) by the motion of the left ventricle during the cardiac cycle. CT of the chest showed a 1.9 × 1.6 cm round and pedunculated mass with soft tissue density on the apical inferior wall of the left ventricle (Fig. 2). The patient was referred to the Department of Thoracic Surgery. On opening the left atrium, there was a mass attached deep to the inferior wall of the left ventricle. Both the mass and stalk were resected. The resected tumor was 1.8 × 1.5 cm and had the appearance of a sea anemone (Fig. 3).
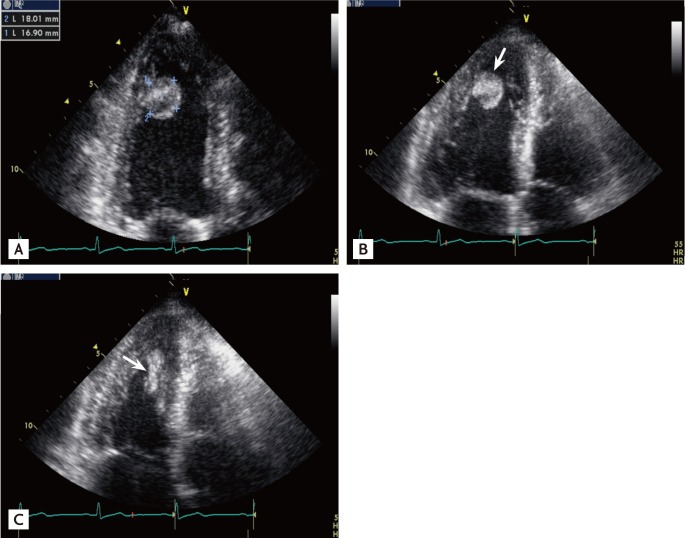
Transthoracic echocardiography reveals a round mass (1.8 × 1.7 cm) with a central echolucent area attached to the apical inferior wall of the left ventricle (A). The shape of the mass changed during the cardiac cycle, such as in diastole (B) and systole (C) (arrows).
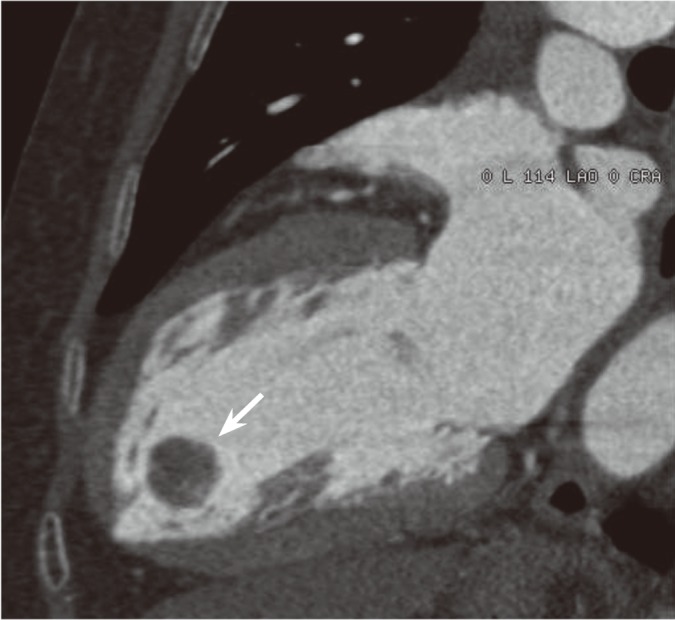
Coronal computed tomography image showing a mass with soft tissue density at the apical inferior wall of the left ventricle (arrow).
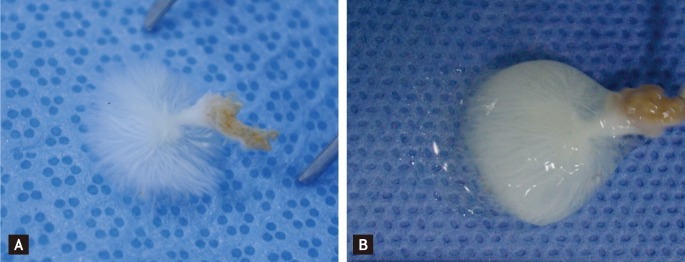
Gross specimen. The tumor had the appearance of a sea anemone in water (A), but out of water (B) looked like a solid mass (1.8 × 1.5 cm) covered with gelatinous material.
Intraoperative transesophageal echocardiography demonstrated the absence of a residual mass. Histology showed that the lesion had a papillary configuration with an avascular connective tissue core (Fig. 4). The postoperative course was uneventful.
DISCUSSION
Among primary cardiac neoplasms, benign tumors account for 63% and malignant tumors for 37% [5]. CPF is a rare and benign primary cardiac neoplasm of unknown prevalence. The mean age at diagnosis of CPF is approximately 60 years, although it has been reported in teenagers and children. CPF affects both sexes, but males predominate in most series [4]. In the study of a series at the Mayo Clinic from 1957 to 2001, of 110 primary cardiac tumors that were surgically removed there were 80 myxomas and only seven CPFs [6]. A recent study reported that CPF represented 5% of primary cardiac tumors in 21 patients [7]. In a report of 110 CPF cases [8], about 90% of CPFs appeared on cardiac valves, and the remainder originated from nearly all portions of the non-valvular endocardium, including the left ventricular septum, the left ventricular mural endocardium, the right ventricular outflow tract, the right atrium, the atrial septum, the papillary muscle, the chordate tendinea, the Eustachian valve, and the Chiari network.
Grossly, CPFs resemble sea anemones. They have a gelatinous membrane on the surface and a stalk with multiple papillary projections. Microscopically, CPFs are composed of a central stalk with radiating villus-like projections. The papillae are avascular structures, containing a core of dense collagen fibers admixed with varying amounts of reticulin and elastin fibers. The epithelial lining is contiguous with the rest of the endocardium. The microscopic structure of a CPF closely resembles that of chordea tendineae, which prompted some to propose a hamartomatous-like pathogenesis [4].
A CPF is a collection of avascular fronds of dense connective tissue lined by endothelium [5]. Therefore, endothelial proliferation is a major component of CPF. However, it is deemed to be a reactive process rather than a true neoplasm [9]. It is suggested that CPF may be associated with Lambl's excrescence and shear stress, and congenital factors may be involved in its development; however, the cause remains unclear [4].
With improved echocardiographic resolution due to higher transducers and new imaging modalities, small and ill-defined intracardiac tumors are being identified increasingly frequently. CPF was considered to be an incidental autopsy finding and to have no clinical significance in the past. However, complications causing life-threatening systemic embolic events have been reported recently. The clinical presentation of a cardiac tumor is determined by its location, size, and mobility. In particular, left-sided, larger sized (≥ 1 cm) and highly mobile tumors have a higher incidence of embolization. CPFs have a predilection for the left side of the heart. In a review of the literature regarding complications in 79 cases of CPF, 61 lesions occurred on the left side of the heart, whereas only 18 were right-sided [4]. CPFs are generally slow growing tumors, but may serve as nidi, allowing formation of large superimposed thrombi over a short period of time. Symptoms include cerebral infarction and transient cerebral ischemic attack associated with soft and fragile fibroelastoma in 44% of patients, angina pectoris in 18%, myocardial infarction in 10%, cardiac failure in 9%, and sudden death in 8% [10].
Symptomatic CPF should be resected surgically; however, there are no guidelines for the treatment of asymptomatic CPF. Large, left-sided mobile tumors should be excised to prevent sudden death and emboli. Small, non-mobile tumors may be followed with serial echocardiography and removed if they increase in size or become mobile or symptomatic. CPFs are usually pedunculated and may easily be removed with associated endocardial tissue. Undoubtedly, surgical resection is curative with excellent short- and long-term prognoses. Moreover, recurrence after surgical removal has not been reported.
Various methods have been proposed for complete excision of left-sided CPFs to avoid a left ventriculotomy due to further complications. For tumors deep within the left ventricular cavity close to the apex, the use of a cardioscope passing through the aortic or mitral valve is recommended to avoid damage to the valvular apparatus. In our case, we used a surgical approach through the left atrium and mildly pushed up the left ventricular apical inferior wall for easy resection of the mass. Recently, a case in which a CPF deep in the left ventricle was resected under the guidance of a gastrointestinal fiberscope was reported.
In this report, we present a case of CPF attached to the left ventricular apical inferior wall, which was successfully removed surgically.
Notes
No potential conflict of interest relevant to this article is reported.
