Use of 18F-FDG PET to predict tumor progression and survival in patients with intermediate hepatocellular carcinoma treated by transarterial chemoembolization
Article information
Abstract
Background/Aims
18F-Fluorodeoxyglucose positron-emission tomography (18F-FDG PET) has been used to assess the biological behavior of hepatocellular carcinoma (HCC). In this study, we investigated the usefulness of 18F-FDG PET for predicting tumor progression and survival in patients with intermediate Barcelona Clinic Liver Cancer (BCLC) intermediate-stage HCC treated by transarterial chemoembolization (TACE).
Methods
From February 2006 to March 2013, 210 patients treated with TACE, including 77 patients with BCLC intermediate-stage HCC, underwent examination by 18F-FDG PET. 18F-FDG uptake was calculated based on the tumor maximum (Tmax) standardized uptake value (SUV), the liver mean (Lmean) SUV, and the ratio of the Tmax SUV to the Lmean SUV (Tmax/Lmean).
Results
The mean follow-up period for the 77 patients (52 males, 25 females; average age, 63.3 years) was 22.2 months. The median time to progression of HCC in patients with a low Tmax/Lmean (< 1.83) and high Tmax/Lmean (≥ 1.83) was 17 and 6 months, respectively (p < 0.001). The median overall survival time of patients with a low and high Tmax/Lmean was 44 and 14 months, respectively (p = 0.003). Multivariate analysis revealed that the Tmax/Lmean was an independent predictor of overall survival (hazard ratio [HR], 1.96; 95% confidence interval [CI], 1.210 to 3.156; p = 0.006) and tumor progression (HR, 2.05; 95% CI, 1.264 to 3.308; p = 0.004).
Conclusions
18F-FDG uptake calculated by the Tmax/Lmean using PET predicted tumor progression and survival in patients with BCLC intermediate-stage HCC treated by TACE.
INTRODUCTION
18F-Fluorodeoxyglucose positron-emission tomography (18F-FDG PET) is well established as a valuable imaging tool for detecting various types of malignancy, such as head and neck, pancreas, brain, lung, colon, and metastatic liver tumors [1,2]. However, the diagnostic accuracy of 18F-FDG PET for the evaluation of primary hepatocellular carcinoma (HCC) is unsatisfactory due to various features of 18F-FDG uptake in HCC. The sensitivity of 18F-FDG-PET for detecting HCC is approximately 50% to 70% [3,4,5], and 18F-FDG uptake by HCC varies according to the size and histological grade of the tumor [6,7]. Furthermore, several studies have reported that the degree of 18F-FDG uptake is an independent prognostic factor in patients with HCC who undergo sorafenib treatment [8]. Monitoring of 18F-FDG uptake using PET may be useful for assessing the biological behavior of HCC [9,10]. Patients with Barcelona Clinic Liver Cancer (BCLC) intermediate-stage HCC represent a heterogeneous population characterized by various degrees of liver dysfunction, tumor burdens, and disease etiologies [11]. According to the BCLC staging system, transarterial chemoembolization (TACE) is the standard treatment for intermediate-stage HCC. However, the long-term survival of patients treated with TACE is not satisfactory [12]; thus, evaluation and monitoring of the treatment response in patients with intermediate-stage HCC is important. Several studies have evaluated the association between the monitoring system used and mortality in patients with HCC treated by TACE [13,14]; however, several limitations of these studies were evident. In the present study, we assessed the usefulness of 18F-FDG PET for evaluating tumor progression and survival in patients with intermediate-stage HCC treated by TACE.
METHODS
We retrospectively analyzed patients with HCC who underwent PET/computed tomography (CT) from February 2006 to March 2013 at Soonchunhyang University Bucheon Hospital and Seoul Hospital. In total, 210 patients were enrolled. For HCC staging, all patients underwent triple-phase CT or contrast-enhanced magnetic resonance imaging (MRI) of the abdomen, chest X-ray, and/or chest CT; a whole-body bone scan; and PET/CT. Diagnosis of HCC was based on pathological confirmation or the typical appearance of HCC on either two dynamic imaging tests (CT and MRI) or on one dynamic imaging test with an elevated serum α-fetoprotein (AFP) level of > 200 ng/mL [15]. The modified International Union Against Cancer tumor node metastasis (TNM) staging system was used to define the tumor stage [16]. The BCLC algorithm was developed using performance status (PS) and liver function to classify tumors into early, intermediate, and advanced. In particular, intermediate-stage tumors were defined as multinodular (> 3) tumors without extrahepatic spread in patients with a PS of 0 and Child-Pugh (CP) class of A or B [17]. In total, 77 patients with intermediate-stage HCC were treated with TACE. Data were collected to examine the patients' characteristics including sex, age, etiology of liver disease, CP classification, AFP level, primary tumor size, model for end-stage liver disease (MELD) score, TNM tumor stage, portal vein tumor thrombosis, Eastern Cooperative Oncology Group PS, presence of ascites, survival time since initial diagnosis, and progression time.
Transarterial chemoembolization
TACE was performed once every 4 to 6 weeks using selective catheterization of the hepatic segmental arteries feeding the tumors. Patients were treated with a mixture of intra-arterial adriamycin (50 mg/m2) and lipiodol (5 to 10 mL) with gelfoam embolization. After embolization, angiography was performed to confirm blood flow to other arterial vessels and determine the extent of vascular occlusion. CT and laboratory findings, including the AFP level, were used to assess the treatment response according to the modified Response Evaluation Criteria in Solid Tumors 4 weeks after TACE [18].
PET imaging
All patients fasted for > 8 hours. When the plasma glucose level reached < 180 mg/dL, an 18F-FDG PET scan was performed. Scanning was initiated approximately 1 hour after intravenous administration of 0.7 MBq/kg 18F-FDG. A low-dose noncontrast CT scan using a PET/CT camera (Siemens Biograph 2, Siemens, Knoxville, TN, USA) was obtained for attenuation correction. Whole-body images were obtained, and data were acquired in three-dimensional (3D) mode after intravenous administration of 18F-FDG. The obtained PET images were interpreted by two experienced nuclear medicine physicians, and decisions regarding the findings were reached by consensus.
The 18F-FDG uptake was calculated based on the tumor maximum (Tmax) standardized uptake value (SUV), the liver mean (Lmean) SUV, and the ratio of the Tmax SUV to the Lmean SUV (Tmax/Lmean). The Tmax SUV was measured based on the maximal radioactivity concentration in the tumor per injection dose using the following equation: Tmax SUV = concentration (kBq/mL) / dose (kBq) / body weight (kg).
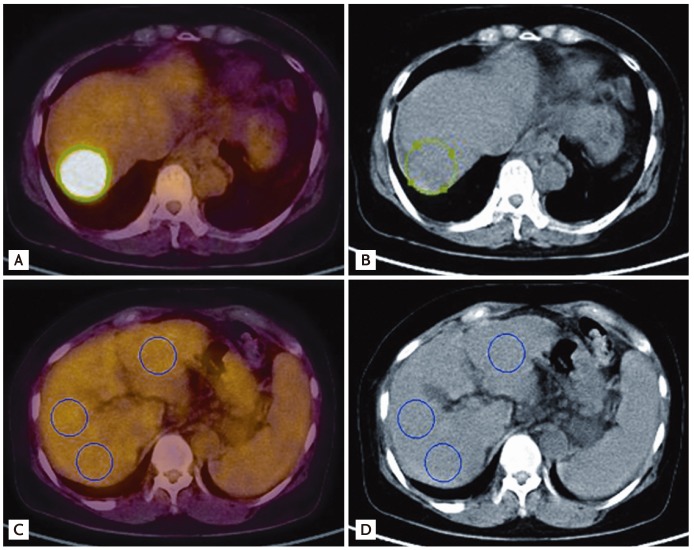
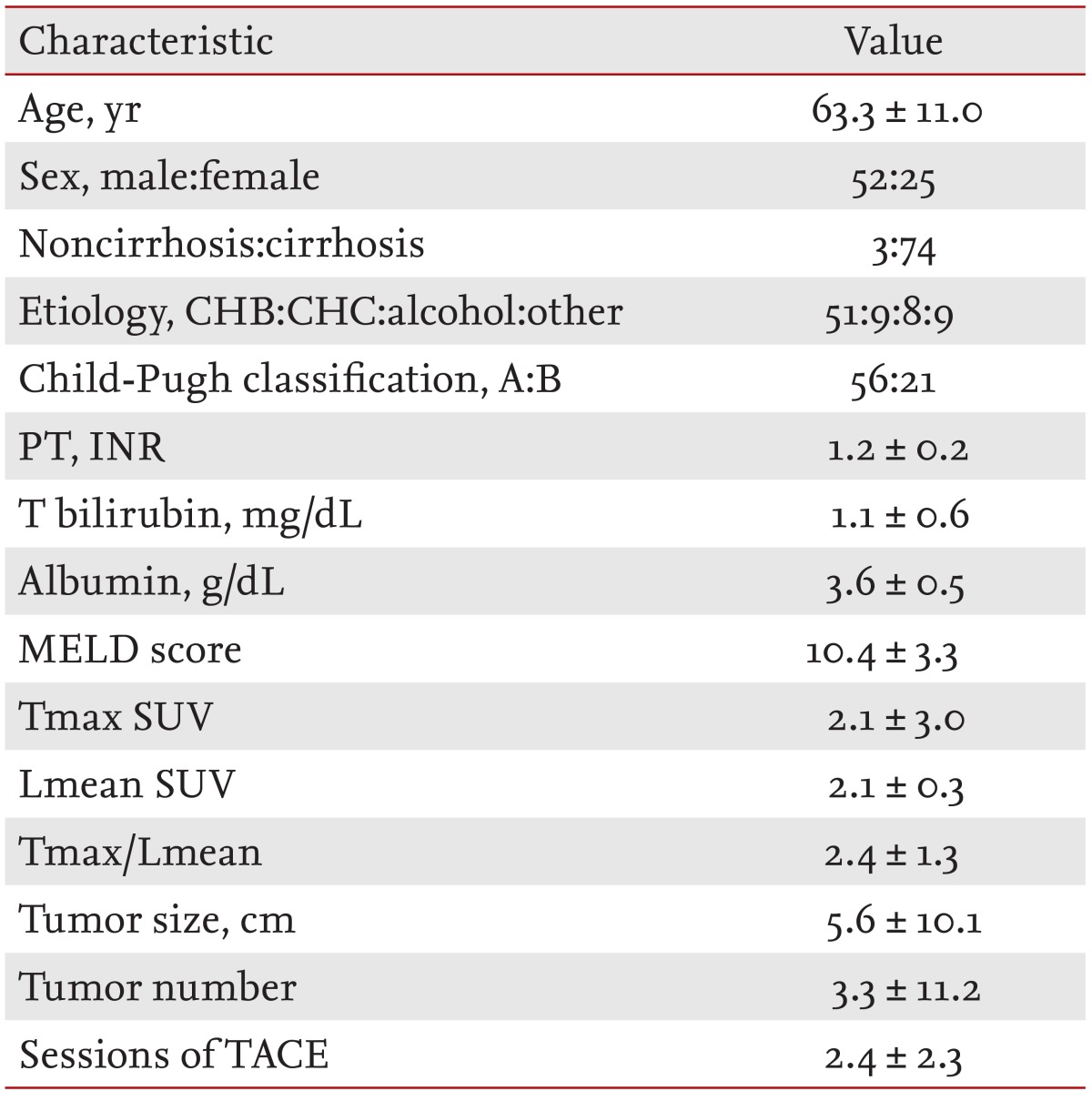
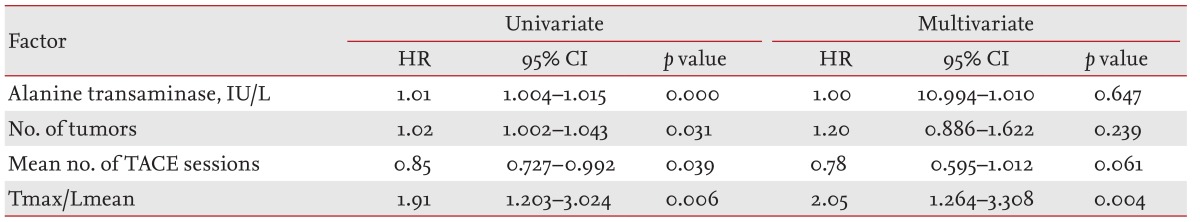
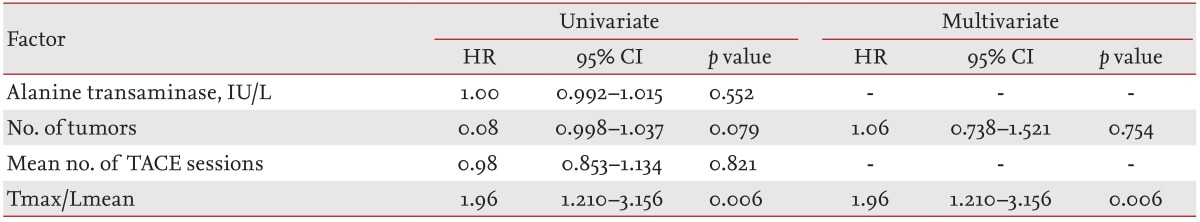
The Lmean was measured using the average SUVs at three points in nontumor tissues. Three-pointed regions of interest (ROIs) were defined on the target lesions on transaxial tomograms of the PET images. The ROIs were established using 3D analysis and drawn on the normal liver (each 100 mm3) (Fig. 1).
Statistical analysis
All statistical analyses were performed using PASW Statistics version 18.0 (SPSS Inc., Chicago, IL, USA). All values are expressed as the means ± standard deviation. The cumulative progression-free survival rate and cumulative overall survival rate were analyzed with a Kaplan-Meier model. The difference was compared using the log-rank test. The statistical significance of the prognostic factors was analyzed using Cox regression in a multivariate model. Variables with a p < 0.05 were considered for the analysis, and 95% confidence intervals (CIs) were calculated.
RESULTS
Baseline characteristics
Seventy-seven patients (52 males, 25 females) with a mean age of 63.3 ± 11.0 years were included in this study. The etiology of liver disease included hepatitis B virus (n = 51, 66.2%), hepatitis C virus (n = 9, 11.6%), alcoholic liver disease (n = 8, 10.4%), autoimmune hepatitis (n = 3, 3.9%), and unknown (n = 6, 7.8%). The CP classification was A in 56 patients (72.7%) and B in 21 patients (27.3%). Seventy-four patients (96.1%) were cirrhotic with an average MELD score of 10.4 ± 3.3, and 16 patients (20.8%) had serum AFP level of > 200 ng/mL. The mean size and number of tumors were 5.6 cm and 3.3, respectively. The patients' baseline characteristics are presented in Table 1.
Factors associated with tumor progression
Forty-five of the 77 patients (58.4%) exhibited tumor progression during the follow-up period. Thirty-two patients with a measured Tmax/Lmean were enrolled. Based on our data, the median Tmax/Lmean (1.83) was used as the cutoff value by which to differentiate the low Tmax/Lmean and high Tmax/Lmean groups. The cumulative incidence of progression according to a low and high Tmax/Lmean is shown in Fig. 2A. The median tumor progression time of patients with a low Tmax/Lmean (< 1.83) and high Tmax/Lmean (≥ 1.83) was 17 and 6 months, respectively (p < 0.001). The statistically significant predictive factors associated with progression-free survival of patients with intermediate-stage HCC using the univariate Cox regression model were the alanine transaminase (ALT) level, number of tumors, mean number of TACE sessions, and Tmax/Lmean. The Tmax/Lmean was an independent risk factor for progression-free survival after adjusting for the mean number of TACE sessions, ALT level, and number of tumors (hazard ratio [HR], 2.05; 95% CI, 1.264 to 3.308; p = 0.004) (Table 2).
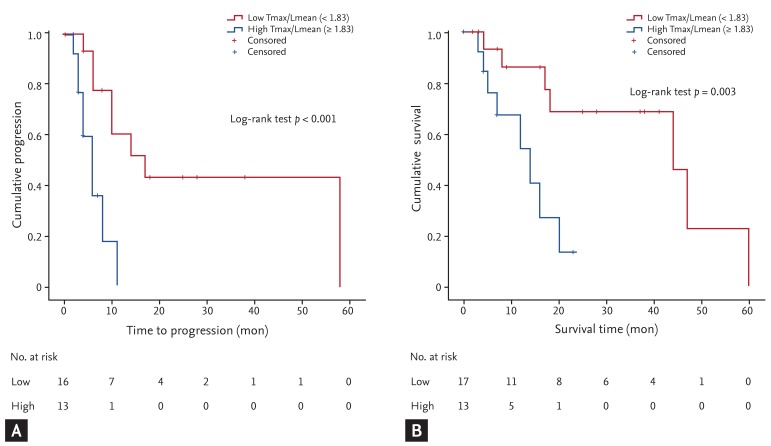
Cumulative incidence of intermediate hepatocellular carcinoma patients with transarterial chemoembolization treatment. (A) Cumulative incidence of progression according to tumor maximum/liver mean (Tmax/Lmean). (B) Kaplan-Meier curve of overall survival according to Tmax/Lmean. Tmax/Lmean = ratio of Tmax standardized uptake value (SUV) to Lmean SUV.
Factors associated with overall survival
During the follow-up period, 30 of the 77 patients (38.9%) died. Thirty-two patients with a measured Tmax/Lmean were analyzed using the Kaplan-Meier curve. The Kaplan-Meier curve of overall survival according to a low and high Tmax/Lmean is shown in Fig. 2B. The median overall survival time of patients with a low Tmax/Lmean (< 1.83) was longer than that in patients with a high Tmax/Lmean (≥ 1.83) (44 and 14 months, respectively; p = 0.003). The clinical factors significantly associated with overall survival of patients with intermediate-stage HCC using the univariate Cox regression model were the number of tumors and the Tmax/Lmean. In the multivariate analysis, Tmax/Lmean was the only significant predictive factor (HR, 1.96; 95% CI, 1.210 to 3.156; p = 0.006) (Table 3).
DISCUSSION
Approximately 20% of all patients with HCC are classified as having intermediate BCLC-stage cancer, a noncurative disease in a heterogeneous population of patients with varying tumor burdens, degrees of liver dysfunction, and disease etiologies [11,12,19], Surgical resection and radiofrequency ablation are not considered to be primary treatment modalities for intermediate-stage HCC. Nevertheless, approximately only 50% of patients with intermediate-stage HCC are treated with TACE in Korea, similar to other countries [20]. Thus, follow-up and monitoring after TACE in patients with intermediate-stage HCC is important.
PET has recently been used for the diagnosis of HCC. Unlike most diagnostic modalities, PET provides information regarding tumor metabolism. Because of differences in the activity of enzymes such as glucose-6-phosphatase, different accumulations of 18F-FDG can be observed in a malignant tumor. The activity of glucose-6-phosphatase reportedly varies widely in patients with HCC; thus, 18F-FDG uptake varies similarly [21,22]. According to previous studies, the detection sensitivity of 18F-FDG PET for HCC is approximately 50% to 70% [3,4,5]. Because of the low 18F-FDG uptake sensitivity in individual HCCs, we selected the HCC ROIs using CT for accurate measurement of Tmax [23].
18F-FDG PET has been used to predict outcomes in patients with HCC. Lee et al. [8] reported that the degree of 18F-FDG uptake is an independent prognostic factor in patients with HCC who undergo sorafenib treatment. Another study reported that HCCs with high uptake of 18F-FDG are associated with a more aggressive outcome and that uptake is related to gene expression [9].
In the present study, 18F-FDG uptake was calculated based on the Tmax SUV, Lmean SUV, and Tmax/Lmean. Specifically, the Lmean SUV was measured as the average SUV from three points in nontumor tissues determined by 3D analysis and drawn on the normal liver (each 100 mm3), unlike other studies [24,25,26]. Other studies have calculated the Lmean SUV by drawing two circular ROIs of both lobes. Because 18F-FDG PET is affected by underlying liver conditions such as liver cirrhosis, the tumor to nontumor SUV ratio is more useful than other parameters used in previous studies [24,27]. Additionally, the Tmax/Lmean was a significant independent factor associated with progression (HR, 2.05; 95% CI, 1.264 to 3.308; p = 0.004) and an independent factor of overall survival (HR, 1.96; 95% CI, 1.210 to 3.156; p = 0.006).
The limitations of this study were the small number of participants and retrospective design. Therefore, selection bias may have affected the results. Various factors-such as the PS after TACE and the time interval between TACE sessions-could have affected the results. However, because TACE was performed using the same protocol at a single institution, this study had relatively little variation. Additionally, this study could not predict the correlation between the degree of treatment response and PET. Nevertheless, we determined that 18F-FDG uptake is likely associated with overall survival and tumor progression. Additional prospective studies with larger cohorts are necessary to confirm these results.
In conclusion, PET can predict tumor progression and survival in patients with intermediate-stage HCC who undergo TACE. Tmax/Lmean was the only significant independent factor for both progression-free survival and overall survival. Thus, 18F-FDG PET is a good outcome predictor for patients with intermediate-stage HCC treated with TACE.
KEY MESSAGE
Evaluation and monitoring of the treatment response in patients with intermediate-stage hepatocellular carcinoma (HCC) is important.
The ratio of the tumor maximum standardized uptake value (SUV) to the liver mean SUV was the only significant independent factor for both progression-free survival and overall survival.
18F-Fluorodeoxyglucose positron-emission tomography for evaluating tumor progression and survival in patients with intermediate-stage HCC treated by transarterial chemoembolization is usefulness.
Acknowledgments
This work was supported by the Soonchunhyang University Research Fund.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.